2024 Global Biopharma Engineering Forum & ISPE China Conference
LEVERGE GLOBAL KNOWLEDGE, ENABLE CHINA EXCELLENCE
Apr 12, 2024
2024
Registration for this event is now closed.
09:00 - 17:00
GMT+8
1712883600The aim of Shanghai ISPE Pharmaceutical Information Co., Ltd. is to transform the international advanced management concepts into technical guidelines and become the gold standard for the pharmaceutical industry in production and quality control through the cooperation with various national drug authorities, enterprises and academic institutions around the world, and to share the advanced experience of the pharmaceutical industry, explore innovative cutting-edge technologies and cutting-edge solutions.
According to the framework of ISPE China Committee, this conference will focus on six academic dimensions, namely Regulation and Compliance, Manufacturing and Engineering, Chemical Drug Production and Process, Biological Drug Production and Process ,Gene and Cell Therapy, and Supply Chain. Under the leadership of Mr. Zhang Ping, President of ISPE China Committee and Chairmen of academic sub-committees, With the authoritative guide of the industry as the core, it tells and shares the hot topics of the industry, targets and focuses on the difficulties and pain points of enterprise development, and makes precise efforts to carry out discussions and gather well-known experts and scholars in the industry to feel the industry dynamics.
Business Cooperate/ Sponsorship
Sponsor Details or Speaker/Topic Recommend, please contact : Lmiaoyi@ispe.org / YCao@ispe.org
Tickets
Standard Individual
RMB 3,000
Member Price
RMB 4,500
Standard Price
Buy TicketMember standard fare: 3000 yuan/person; Non-member standard fare: 4500 yuan/person; Registrations before February are 40% off and registrations in March are 20% off
To join: www.ispe.cnParticipants - Institutions and students
RMB 1,500
Member Price
RMB 1,800
Standard Price
Buy TicketAfter filling in the registration, the registration will be approved within 1-3 working days. If the approval is approved, you will receive a confirmation letter from no-reply@glueup.com to your registered email address.
If not, please check the spam. If not, please contact email: lmiaoyi@ispe.org or phone/wechat: 17721318289多人参会 - 标准
RMB 3,000
Member Price
RMB 4,500
Standard Price
Buy Ticket请联系LMiaoyi@ispe.org获取折扣码
在填写完注册报名以后,于1-3个工作日审批通过,如果审批通过,您的注册邮箱将收到由no-reply@glueup.com发出的活动确认函,
如收件箱中没有,烦请查看垃圾邮件,如没收到请联系邮箱:lmiaoyi@ispe.org 或电话/微信:17721318289线上参会
RMB 1,500
Member Price
RMB 1,800
Standard Price
Buy Ticket线上会议链接;关注微信公众号:ISPE 埃思博益 - 菜单栏 - 年会信息 - 现场直播
Exhibitor Passes
Complimentary
Standard Price
Reserve TicketNo materials, no lunch, Exhibition area free, conference area charge
Agenda
- 主会场 Main Forum
- Track 1
- ISPE会员活动 ISPE Membrers Session
- 生物制药分会场 Biological Drug Forum
- 基因与细胞治疗分会场 GCT Forum
- 化学制药分会场 Chemistry Drug Forum
- 工程设计与项目管理分会场 Engineering design and project management Forum
- 生产工程分论坛 Manufacturing Engineering Forum
- 合规分会场 Regulation & Compliance Forum
- 创新投资分论坛 Innovation Investment Forum
08
00
-
09
00
09
00
-
09
05
09
05
-
09
25
09
25
-
09
45
ISPE CEO致欢迎辞 (Welcome Speech by ISPE CEO )
Thomas B. Hartman09
55
-
10
00
10
00
-
10
20
10
20
-
10
50
10
50
-
11
20
Friday, April 12, 2024
13
30
-
21
30
Saturday, April 13, 2024
09
00
-
09
15
致欢迎辞 Welcome Speech
王麟Lynn09
15
-
09
50
09
50
-
10
25
10
25
-
10
50
10
50
-
11
25
12
00
-
12
10
12
10
-
13
00
13
00
-
13
15
13
15
-
13
50
14
05
-
14
40
15
15
-
15
45
16
15
-
16
25
Friday, April 12, 2024
18
00
-
18
05
18
05
-
18
15
18
50
-
19
00
19
10
-
19
30
19
45
-
19
50
Friday, April 12, 2024
13
00
-
13
15
13
30
-
14
15
15
00
-
15
30
15
30
-
16
15
16
15
-
16
25
Saturday, April 13, 2024
09
00
-
09
15
09
15
-
09
50
ISPE指南介绍:活性化合物的遏制 Introduction for ISPE Good Practice Guide: Containment for Potent Compound
Mr. Maurice B. Parlane , B Tech MIT10
20
-
10
50
10
50
-
11
05
11
05
-
11
35
12
15
-
12
20
Saturday, April 13, 2024
09
00
-
09
10
09
10
-
09
45
09
45
-
10
20
10
20
-
10
50
11
25
-
12
00
12
00
-
12
10
12
10
-
13
00
13
00
-
13
10
13
45
-
14
25
14
25
-
15
00
15
00
-
15
30
16
05
-
16
40
16
40
-
16
50
Friday, April 12, 2024
13
00
-
13
10
13
10
-
13
25
14
15
-
15
05
15
05
-
15
35
15
35
-
16
10
ISPE指南:基于风险的药品生产第2版 ISPE Guidance: Risk-Based Manufacture of Pharma Products 2nd Editio 视频
Ms. Stephanie A. Wilkins, PE17
10
-
17
20
Saturday, April 13, 2024
13
00
-
13
10
会议主席开场致辞 Opening speech by Chair
王卫兵 Alex14
05
-
15
00
15
00
-
15
30
15
30
-
16
15
17
00
-
17
10
Friday, April 12, 2024
13
00
-
13
20
13
20
-
14
05
ISPE GEP指南介绍 ISPE GEP Guideline Introduction
Chip Bennett14
55
-
15
05
15
05
-
15
35
15
35
-
16
05
16
05
-
16
15
Saturday, April 13, 2024
09
00
-
09
10
09
10
-
09
50
最新ISPE清洁验证指南解读 Guide: Cleaning Validation Lifecycle - Applications, Methods, & Controls
Ms. Catherine T. Oakes09
50
-
10
00
10
00
-
10
30
11
00
-
11
30
12
00
-
12
10
12
10
-
13
00
13
00
-
13
10
13
10
-
13
40
Operational Readiness
John Vaughn14
20
-
15
00
15
00
-
15
30
16
10
-
16
40
16
40
-
16
50
Friday, April 12, 2024
13
00
-
13
10
15
00
-
15
30
15
30
-
16
30
17
30
-
17
40
Friday, April 12, 2024
17
30
-
17
40
会议主席致欢迎辞 Welcome Speech by Chair
庞飞飞 Sophie17
40
-
18
10
18
20
-
18
30
18
30
-
18
40
18
40
-
18
50
18
50
-
19
00
19
00
-
19
10
总结 ,会议结束 Summary , Meeting Adjourned
庞飞飞 Sophie致欢迎辞 Welcome Speech
Speakers

王麟Lynn
Head of Project and Alliance at 药明合联 WuXi XDC Co. Ltd会议主席致欢迎辞 Welcome Speech by Chair
Speakers

李锦才博士
CEO of 药明合联会议主席致欢迎辞 Welcome Speech by Chair
Speakers

高杨 博士
首席战略官(CSO) at 上海邦耀生物科技有限公司高杨博士目前担任上海邦耀生物科技有限公司的首席战略官,全面负责公司基因和细胞治疗产品的开发、申报、项目资产管理等工作,协助CEO整个公司的经营
加入上海邦耀生物科技有限公司之前,高博士在制药行业拥有近20年的管理和开发经验,先后在国家药品监管机构、跨国制药企业、生物科技公司和全球CRO公司担任技术/管理工作,熟悉制药行业全链条的运转之道,尤其在药物开发策略、申报策略、质量体系建设和商业化战略方面有着全面、丰富的经验。曾是中国国家药品监督管理局药品审评中心的高级审评官,8年时间内审评过千份申报项目并执行过几十次现场检查,涵盖跨适应症领域的新药临床申请、新药上市申请、仿制药申请、上市后补充申请等技术审评工作,对监管科学和CDE、FDA、EMA及ICH的技术指南有着全面深刻的理解。之后4年担任礼来公司的法规CMC和医疗器械负责人,领导多个创新药CMC策略、技术转移策略和医疗器械注册策略的制定和执行,参与礼来全球加快研发的改革工作,并为礼来在华合作伙伴的创新药研发和商业化提供技术支持。同期兼任RDPAC的CMC委员会轮值主席,领导7个工作组为2015年启动的中国药监改革积极建言献策,为构建支持药物创新的新生态系统做出了杰出贡献。曾担任苏州亚盛制药的法规事务总监,制定并执行中美研发和注册策略,成功获批中、美多个IND。之后担任PAREXEL的首席药物研发和法规顾问,3年时间内为多达30家国内外生物制药公司约50个项目提供研发和监管策略的咨询服务,成功助力他们在中美获得监管部门的批准并开展新药研发。业余时间,高博士积极探索和实践人工智能和数字技术赋能药物研发,设计并开发远程智能化临床试验平台。
会议主席致欢迎辞 Welcome Speech by Chair
Speakers

甘益民
集团副总经理/子公司总经理 at 上海复旦张江生物医药股份有限公司30年制药企业生产运营管理经验。先后在外资制药企业、民营制药企业及上市制药企业担任生产经理、集团副总经理及子公司总经理。
多次到欧、美、日等国家学习制药行业生产运营管理。对制药行业现代管理有深刻理解和丰富的实践经验。荣获上海市实施发明成果优秀企业家,区拔尖人才。
ISPE先进治疗产品-自体细胞治疗指南”指导原则讲解 Introduction for Guide: ATMPs - Autologous Cell Therapy
Speakers

Jeff Odum
Practice Leader: ATMPs & Biologics at Genesis AECJeff Odum’s career encompasses over 30 years of experience in the development, execution, delivery, and management of facility and operational programs in the biotechnology and pharmaceutical industries. During his career, he has been involved in leading and managing strategic planning, feasibility and conceptual planning/design, design, construction, start-up, and validation support, in addition to participating in the development and presentation of licensing packages to the FDA. Specific responsibilities have included positions as Global Lead, Operations Director, Design Manager, Facility/Engineering Manager, Owner’s Representative, Program Manager, and Construction Site Manager for projects focused on the manufacture of human therapeutics including vaccines, cancer therapeutics, generics, and ATMPs. These efforts ranged in size from $1 million to more than $2 billion in total program costs.
Jeff was the North American Education Advisor to ISPE, where he also is currently the Chair of the Global Biotechnology Community of Practice and the Project Lead for the ATMP Guide for Autologous Cell Therapy Products, co-Lead for the new revision of the Biotech Manufacturing Facilities Baseline Guide, chapter author for the Biotech Product and Process Development Baseline Guide, and a lead author for the BPOG Closed Systems Closure Playbook. He has written over 50 Technical articles and published four Industry reference books.
He is a member of the BTEC teaching faculty in NC State University’s Gradute program in Biomanufacturing.
最新ISPE清洁验证指南解读 Guide: Cleaning Validation Lifecycle - Applications, Methods, & Controls
Speakers

Ms. Catherine T. Oakes
Managing Director of Oakes Group Global Ltd.Catherine Oakes is an internationally recognized quality and compliance professional with 30+ years of diverse experience in the regulated healthcare and life sciences industries. She has held many senior and executive management positions in clinical laboratory, pharmaceutical, biotech, diagnostic, and medical device establishments in the U.S. and around the globe. Catherine is the Managing Director and Principal Consultant for Oakes Group Global where she works on the front line with industry, professional associations, health agencies, and regulatory authorities worldwide. Catherine holds a Bachelor of Health Services Administration from Florida Atlantic University and a Master of Science in Jurisprudence from Seton Hall University School of Law with a dual concentration in Pharmaceutical & Medical Device Law & Compliance and Health & Hospital Law.
Digital Systems_Expectations and Value Proposition for Clinical Supply Chain 数字系统_临床供应链的期望和价值主张
Speakers

Glasser Barrett
Senior Director, Global Clinical Supply Chain of TakedaWith more than 25 years of experience working in biotech and life sciences, Barrett has long been passionate about the many applications of digital technology to drug development and supply chain domains. After starting his career in technology consulting at Pfizer, he moved into supply chain planning and integration at Biogen for a number of years before joining Takeda and eventually taking on his current role as a Digital Global Clinical Supply Chain leader. During that time, Barrett obtained his MBA from Boston University and his Master of Public Health from Harvard. When not working, Barrett enjoys music, cooking, and spending time with his dog.
ISPE指南介绍:活性化合物的遏制 Introduction for ISPE Good Practice Guide: Containment for Potent Compound
Speakers

Mr. Maurice B. Parlane , B Tech MIT
Principal/Director of New Wayz Consulting Ltd/CBE Pty LtdMaurice Parlane is Principal of New Wayz Consulting Ltd in New Zealand and a Director of CBE Pty Ltd in Australia. He is a professional engineer with 30 years’ experience within the biopharmaceutical industry, including 20 years as an industry consultant providing support to manufacturing and compliance management; validation and operational excellence projects in Australasia and the Asia Pacific region. Prior to this, he held senior engineering and manufacturing roles within the Glaxo group of companies. He has a Bachelor of Manufacturing Technology (Hons) as well as mechanical and electrical engineering qualifications. Maurice is past president and current director of the ISPE Australasian Affiliate. He is the co-lead of ISPE’s Asia Pacific Regulatory and Quality Harmonization committee and leader of the Process Validation Team and is a member of the Guidance Documents Committee. He is an ISPE PV Instructor and was named ISPE Member of the year in 2016. He is currently serving as the Leader of the ISPE Process Validation Team.
美国食品药品监督管理局的检查计划:基于风险的设施评估方法 US FDA's Inspection Program: A Risk Based Approach to Facility Assessments
Speakers

Zhihao Peter Qiu
External Advocacy Lead APAC at Roche Genentech.裘博士曾任美国FDA CDER药品生产评估办公室生物技术制造部门负责人。在CDER,他负责监督生物制品许可申请的生产控制和设施的科学审查和质量评估。他的部门还负责对CDER监管的生物制品进行许可前/批准前检查。裘博士是全球领先的生物技术生产微生物控制和无菌保证评估专家。在加入CDER之前,他还曾在美国FDA生物制品评估与研究中心(CBER)担任CMC设施审查员/检查员,并在器械与放射卫生中心(CDRH)担任毒理学副主任。
裘博士毕业于南加州大学,获得生物科学博士学位,在加入Roche Genetech之前,曾在信达生物制药(苏州)有限公司担任首席质量官。
Dr. Qiu was a Division Director in the Division of Biotechnology Manufacturing, Office of Pharmaceutical Manufacturing Assessment, CDER, USFDA. At CDER, he oversaw the scientific review and quality evaluation of the manufacturing controls and facilities for Biologics license applications. His Division was also responsible for conducting pre-license/pre-approval inspections for CDER regulated biological products. Dr. Qiu is a global leading expert in microbial controls and sterility assurance assessment for biotech manufacturing, and also has extensive knowledge of FDA regulatory requirements in manufacturing facility assessments and GMP compliance activities. Prior to CDER, he also worked in FDA’s Center for Biologics Evaluation and Research (CBER) as a CMC facility reviewer/inspector and in the Center for Devices and Radiological Health (CDRH) as an associate director for Toxicology.
Dr. Qiu graduated from the University of Southern California with a Ph.D. in Biological Sciences and worked at Innovent Biologics (SuZhou) as the Chief Quality Officer before joining Roche Genentech.
持续工艺监控和改进 Continued process monitoring and improvement
Speakers

温源
全球生产部MFG1和MFG4的负责人 at 药明生物Dr. Yuan Wen is the head of MFG1 and MFG4 of Global Manufacturing of WuXi Biologics. His team was the first in Asia to implement the 4000L single-use bioreactor in GMP and commercial manufacturing approved by ANVISA and EMA. In addition, his team led China’s first 2000L-scale GMP production for mAb using the concentrated fed-batch (CFB) process. Prior to his role in MFG, he was responsible for Cell Culture Process Development at the Wuxi site, with a focus on late phase development and process characterization. Dr. Wen is also an experienced CMC leader to support activities from IND to commercial manufacturing.
Before joining WuXi Biologics, he worked at Life Technologies and Thermo Fisher Scientific in the US, with increasing technical and business responsibilities.
He holds a B.S. degree in Bioengineering and from Chu Kochen Honors College at Zhejiang University, and a Ph.D. degree in Chemical and Biomolecular Engineering from The Ohio State University.
温源博士是药明生物全球生产部MFG1和MFG4的负责人。他的团队是亚洲第一个在GMP商业化生产中使用4000L一次性生物反应器的团队,并获得ANVISA和EMA的批准。他的团队还领导了中国首个2000L规模的单抗GMP生产,采用了浓缩补料(CFB)工艺。在加入MFG之前,他负责无锡基地的细胞培养工艺开发,重点是后期开发和工艺表征。温博士也是一位经验丰富的CMC负责人,支持从IND到商业化生产的活动。
在加入药明之前,他曾在美国的Life Technologies和Thermo Fisher Scientific工作,承担了越来越多的技术和商务责任。
他毕业于浙江大学生物工程和竺可桢荣誉学院,并有俄亥俄州立大学化学和生物分子工程博士学位。
标准化和自动化对于提高产品合规性与生产效率的作用与意义 How standardization and automation improve compliance and productivity
Speakers

Goebel Martin
Sales Director Analytics of Knick GmbH, GermanyPersonal Introduction: With a master degree in chemical engineering from University of Karlsuhe/Germany, Martin started as sales and project engineer for process water and ultra pure plants on international projects. After 5 years working in South East Asia on process water projects he moved to the analytical field focussing on TOC analyzers for ultra pure pharma water and process water for various industries. Martin was involved in many validation processes for TOC analyzer integration into pharma water plants in Europe. Since 5 years Martin is sales director with Knick, Berlin, Germany and responsible for integration of pH analyzers into industrial processes. His special field are applications in bio-pharma industry.

马强
Sales Manager - Analytics at Knick (Shanghai) Electronic Measurement Trading Co., Ltd马强毕业于上海大学测量技术与仪器专业,有10年的分析系统工程工作经验,在西门子、ABB、Servomex担任技术工程师和过程设计师,9年在科伲可、PR电子等公司担任分析仪和自动化产品的技术销售支持和销售。他在科伲可工作了4年,担任分析产品的应用工程师和销售经理,在产品应用和客户服务方面都有丰富的经验。
With bachelor degree of Measurement technique and instrumentation graduated from Shanghai University, Ma Qiang has: 10 years working experience for analytical system engineering as technical engineer and process designer in Siemens, ABB, Servomex. 9 years working experience as technical sales support and sales for analyzers and automation products in Knick, PR electronics etc. He has worked in Knick for 4 years as application engineer and sales manager of analytics products with lot of experience both for product applications and customer service.
清洁验证中最差条件选择与评估 Worst case assessment in cleaning validation
Speakers

牛萍
齐鲁制药 at 质量与合规高级顾问制药工程硕士,执业药师,高级工程师(教授级)。从事制药行业质量管理和质量控制三十多年,曾在国内多家著名制药企业(外企及国企)担任质管部部长、质量总监等职。多次接受国内GMP检查、欧盟及美国FDA检查,并获得优异成绩。曾赴荷兰和法国制药工厂,协助中国药监局完成海外GMP检查。参与了药监局组织的“药品生产验证指南”等文件编写工作。 Master of Pharmaceutical Engineering. Licensed Pharmacist, Senior Engineer (professional level). Worked in pharmaceutical quality management and quality control in the pharmaceutical industry for more than 30 years. Served as Quality Manager and Quality & Compliance Director at some famous Pharmaceutical Company. Received domestic GMP inspection, the EU and FDA CGMP inspection many times, and obtained excellent results. Has been to pharmaceutical plants in the Netherlands and France to assist the China Drug Administration in completing overseas GMP inspections. Involved to write GMP related guidelines such as 《 Pharmaceutical Manufacturing Validation Guideline》etc., which organized by China NMPA.
ICH Q5A 法规更新后对生物安全检测的未来展望
Speakers

吴云飞
技术与法规经理 at Merck目前负责支持默克BioReliance® 生物医药检测服务中国区的细胞基因治疗业务技术与法规事务。她曾专注于抗体类药物细胞培养板块工艺研发及生产。发表过多篇科学论文,专注于分子生物学。她拥有利物浦大学博士学位。
Yunfei Wu is responsible for supporting technology and regulatory affairs associated with BioReliance® Biosafety Testing Services in China, especially for cell and gene therapy. Yunfei worked in the process development of antibody-based therapy, especially for cell culture process development and manufacture. She published several scientific papers, focusing on molecular biology and graduated with a PhD from University of Liverpool.
自我注射系统及新材料预灌封系统在注射剂上的应用 Application of self-injection system and polymer materials in injections
Speakers

郭融 Willian
Vice President, R&D of Shanghai Innopac Medical Technology Co.,LtdWilliam Guo, Vice President R&D for Shanghai Innopac Medical Technology Co.,Ltd.
生物制药清洁验证经验分享 Biotechnology cleaning validation good practice sharing
Speakers

Richard CHAI
Senior Technical Service Manager at STERIS Richard Chai is a Senior Technical Service Manager for the Life Sciences Division of STERIS Corporation. He is an industry speaker at ISPE events (Singapore, Malaysia, Indonesia, Thailand, Philippines, India) and Interphex Japan
Richard先生目前任职STERIS生命科学部门的高级技术服务经理,多次在制药行业会议和展会上作为讲师参与技术交流(如ISPE新加坡、马来西亚、印度尼西亚、泰国、菲律宾、印度和Interphex日本)
Prior to joining STERIS, Richard had 13 years of manufacturing and validation experience working in various pharmaceutical companies. In addition, he has extensive experience in cleaning validation having worked as cleaning validation consultant in various biopharmaceutical and biotech companies
加入STERIS之前,Richard先生有超过13年在制药行业的生产和验证工作的经验,包括曾在多家生物制药公司担任清洗验证咨询师,对许多制药客户在设备验证、产品接触表面的清洗验证等方面提供技术支持
国产生物创新药出海美国策略分享
Speakers

王刚
执行董事、高级副总裁兼首席质量官 at 上海君实生物工程有限公司 Shanghai Junshi Biotechnology Co., Ltd王刚博士现任上海君实生物执行董事、高级副总裁兼首席质量官,全面负责公司生产和研发质量及相关工作。加入上海君实生物前,曾任上海药明生物副总裁,负责质量和全球监管事务。王刚在美国 FDA 和中国 CFDA 工作 13 年,先后任 CFDA 药品审评中心 (CDE) 负责合规和检查的首席科学家,FDA CDER 合规办公室生产质量办公室资深政策顾问,FDA 驻华办公室助理主任,FDA CBER 合规和生物产品质量办公室资深审评员和主持检查员。王刚是经FDA 专业同行评定的生物产品 GMP 领域的专家,在单抗及细胞和基因治疗产品监管及质量管理方面有较高造诣。王刚本科毕业于南京大学生物化学专业,并获得美国 DartmouthMedical School 药理学与毒理学博士学位。博士毕业后在美国国家卫生研究院 (NIH) 国家癌症研究所 (NCI) 从事肿瘤免疫疗法领域的博士后研究,随后在美国德克萨斯州大学 MDAnderson Cancer Center 任助理教授和课题负责人。
Dr. Wang Gang is the Executive Director, Senior Vice President and Chief Quality Officer of
Shanghai Junshi Biosciences. At this role, he is responsible for the company's manufacturing
and R&D quality and related work. Prior to joining Junshi, Dr. Wang was the Vice President of
WuXi Biologics (Shanghai), responsible for quality and global regulatory affairs. Dr. Wang has
13 years of experience in the U.S. FDA and China's CFDA. He has served as Chief Scientist for
Compliance and Inspection in the Center of Drug Evaluation (CDE), CFDA; Senior Policy
Advisor in the Office of Manufacturing Quality in the Office of Compliance, CDER, FDA;
Assistant Country Director of the FDA China Office; senior reviewer and lead inspector in the
Office of Compliance and Biologics Quality, CBER, FDA. Dr. Wang is an FDA peer-reviewed
expert in the field of GMP for biologics, with expertise in regulation and quality management
of monoclonal antibody and cellular and gene therapy products. Dr. Wang graduated from
Nanjing University in biochemistry in China and received his PhD in pharmacology and
toxicology from Dartmouth Medical School in USA. He completed his postdoctoral research
in immunotherapy of cancer at the Surgery Branch of National Cancer Institute (NCI), National
Institutes of Health (NIH). He was an assistant professor and principal investigator at the MD
Anderson Cancer Center of the University of Texas prior to joining the FDA.
AI赋能医药智造的最新实践 The latest practices of Al in smart manufacturing
Speakers

陶静雯
量子创新场主任 at 明度智云硕士毕业于英国牛津大学。现任职于明度智云量子创新场,主要负责人工智能在医药行业的应用探索和实施。研究方向包括药物研发、质量控制、生产过程以及合规性问题,涉及搭建AI药物属性预测平台、利用AI赋能的mRNA疫苗传递系统设计平台、AI药品生产异常检测平台以及AI图像检测平台,工作范围覆盖从算法设计到应用实践。
Graduated with a master's degree from the University of Oxford. Currently serves in the AI Innovation Department of Mingdu Smart Cloud, primarily responsible for the exploration and implementation of artificial intelligence-enabled applications in the pharmaceutical industry. Research areas include drug R&D, quality, production, and compliance scenarios, responsible for building an AI drug property prediction platform, an AI-enabled mRNA vaccine delivery system design platform, an AI drug production anomaly detection platform, and an AI image detection platform, covering everything from algorithm design to practical application.
清洁验证在中药生产中的实施 Cleaning validation in Chinese medicine manufacturing
Speakers

孙英强
质量经理 Quality Manager at 步长药业/Buchang PharmaceuticalPersonal introduction:Mr. Sun Yingqiang, the quality manager of Buchang Pharmaceutical, has been engaged in the production quality management of traditional Chinese medicines (oral preparations and traditional Chinese medicine injections) for a long time. He is an expert in building systems and promoting the improvement of quality management. He has hosted numerous regulatory on-site inspections for sterile products, and has accumulated rich practical experience. Specially appointed lecturer of the Medical Association, and participated in the editorial board of the university textbook "Drug Analysis". As a representative of the enterprise, I have participated multiple times in the drafting and revision of national regulations, such as the Special Provisions on the Supervision of Traditional Chinese Medicine Production.
孙英强先生,步长制药质量经理,长期从事中成药(口服制剂与中药注射剂)生产质量管理,擅长中成药质量体系搭建和推动质量管理水提升,多次接受无菌产品的现场检查,积累了丰富的实战经验。医药协会特聘讲师,作为编者出版高校教材《药物分析》,作为企业代表多次参与国家法规指南,如《中药生产监督专门规定》等的编写修订工作。
新型一次性碟片式离心机在收获工艺环节的应用 Maximizing Efficiency and Cost-saving: Thermo Fisher Single-use Centrifuge for harvest process
Speakers

张龙浩
生物工艺部技术支持经理 at 赛默飞世尔科技赛默飞生物工艺部一次性生物工艺技术经理,在工艺过程控制领域拥有超过13年的行业经验,2018年加入赛默飞生物工艺担任产品技术专家,专注于一次性技术和自动化解决方案,负责一次性生物反应器、一次性工艺容器以及一次性层析系统和一次性离心机等工艺装备在生物工艺上下游的应用和推广,参与过多家头部CDMO以及生物药企一次性生物反应器GMP生产线的新建和运行。
企业对高活化合物的综合管理 Management of highly potent compounds by pharmaceutical companies
Speakers

黄玮
总裁 President of 上海复宏汉霖生物制药有限公司 Shanghai Henlius Biopharmaceutical Co.,Ltd.黄玮女士做为公司总裁,全面负责生产与工程部全面负责商业化产品的生产供应及松江基地的建设,同时带领质量团队打造国际化质量标准体系和运行。
黄玮女士拥有华东理工大学生物化学工程学士学位及马里兰大学化学与生化工程硕士学位。在加入复宏汉霖之前,黄玮女士在制药和生物技术行业拥有30年的高级管理和领导经验,包括工艺开发、技术转让、制造、工艺和设施设计、资本项目执行和质量体系实施。
As president of the company, Wei leads the manufacturing, quality, MSAT, clinical supply, engineering, supply chain and other operations functions. Wei holds a Bachelor's degree in biochemical engineering from East China University of Science and Technology and a Master's degree in chemical and biochemical engineering from the University of Maryland. Before joining Henlius, Wei held several senior management and leadership positions in pharmaceutical and biotechnology industry. She has thirty years’experience in process development, technology transfer, manufacturing, GMP facility design, and quality system implementation.
总结 ,会议结束 Summary , Meeting Adjourned
Speakers

王麟Lynn
Head of Project and Alliance at 药明合联 WuXi XDC Co. Ltd总结 ,会议结束 Summary , Meeting Adjourned
Speakers

高杨 博士
首席战略官(CSO) at 上海邦耀生物科技有限公司高杨博士目前担任上海邦耀生物科技有限公司的首席战略官,全面负责公司基因和细胞治疗产品的开发、申报、项目资产管理等工作,协助CEO整个公司的经营
加入上海邦耀生物科技有限公司之前,高博士在制药行业拥有近20年的管理和开发经验,先后在国家药品监管机构、跨国制药企业、生物科技公司和全球CRO公司担任技术/管理工作,熟悉制药行业全链条的运转之道,尤其在药物开发策略、申报策略、质量体系建设和商业化战略方面有着全面、丰富的经验。曾是中国国家药品监督管理局药品审评中心的高级审评官,8年时间内审评过千份申报项目并执行过几十次现场检查,涵盖跨适应症领域的新药临床申请、新药上市申请、仿制药申请、上市后补充申请等技术审评工作,对监管科学和CDE、FDA、EMA及ICH的技术指南有着全面深刻的理解。之后4年担任礼来公司的法规CMC和医疗器械负责人,领导多个创新药CMC策略、技术转移策略和医疗器械注册策略的制定和执行,参与礼来全球加快研发的改革工作,并为礼来在华合作伙伴的创新药研发和商业化提供技术支持。同期兼任RDPAC的CMC委员会轮值主席,领导7个工作组为2015年启动的中国药监改革积极建言献策,为构建支持药物创新的新生态系统做出了杰出贡献。曾担任苏州亚盛制药的法规事务总监,制定并执行中美研发和注册策略,成功获批中、美多个IND。之后担任PAREXEL的首席药物研发和法规顾问,3年时间内为多达30家国内外生物制药公司约50个项目提供研发和监管策略的咨询服务,成功助力他们在中美获得监管部门的批准并开展新药研发。业余时间,高博士积极探索和实践人工智能和数字技术赋能药物研发,设计并开发远程智能化临床试验平台。
总结 ,会议结束 Summary , Meeting Adjourned
Speakers

甘益民
集团副总经理/子公司总经理 at 上海复旦张江生物医药股份有限公司30年制药企业生产运营管理经验。先后在外资制药企业、民营制药企业及上市制药企业担任生产经理、集团副总经理及子公司总经理。
多次到欧、美、日等国家学习制药行业生产运营管理。对制药行业现代管理有深刻理解和丰富的实践经验。荣获上海市实施发明成果优秀企业家,区拔尖人才。
总结 ,会议结束 Summary , Meeting Adjourned
Speakers

李锦才博士
CEO of 药明合联会议主席致欢迎辞 Welcome Speech by Chair
Speakers

王麟Lynn
Head of Project and Alliance at 药明合联 WuXi XDC Co. Ltd会议主席致欢迎辞 Welcome Speech by Chair
Speakers

高杨 博士
首席战略官(CSO) at 上海邦耀生物科技有限公司高杨博士目前担任上海邦耀生物科技有限公司的首席战略官,全面负责公司基因和细胞治疗产品的开发、申报、项目资产管理等工作,协助CEO整个公司的经营
加入上海邦耀生物科技有限公司之前,高博士在制药行业拥有近20年的管理和开发经验,先后在国家药品监管机构、跨国制药企业、生物科技公司和全球CRO公司担任技术/管理工作,熟悉制药行业全链条的运转之道,尤其在药物开发策略、申报策略、质量体系建设和商业化战略方面有着全面、丰富的经验。曾是中国国家药品监督管理局药品审评中心的高级审评官,8年时间内审评过千份申报项目并执行过几十次现场检查,涵盖跨适应症领域的新药临床申请、新药上市申请、仿制药申请、上市后补充申请等技术审评工作,对监管科学和CDE、FDA、EMA及ICH的技术指南有着全面深刻的理解。之后4年担任礼来公司的法规CMC和医疗器械负责人,领导多个创新药CMC策略、技术转移策略和医疗器械注册策略的制定和执行,参与礼来全球加快研发的改革工作,并为礼来在华合作伙伴的创新药研发和商业化提供技术支持。同期兼任RDPAC的CMC委员会轮值主席,领导7个工作组为2015年启动的中国药监改革积极建言献策,为构建支持药物创新的新生态系统做出了杰出贡献。曾担任苏州亚盛制药的法规事务总监,制定并执行中美研发和注册策略,成功获批中、美多个IND。之后担任PAREXEL的首席药物研发和法规顾问,3年时间内为多达30家国内外生物制药公司约50个项目提供研发和监管策略的咨询服务,成功助力他们在中美获得监管部门的批准并开展新药研发。业余时间,高博士积极探索和实践人工智能和数字技术赋能药物研发,设计并开发远程智能化临床试验平台。
会议主席致欢迎辞 Welcome Speech by Chair
Speakers

甘益民
集团副总经理/子公司总经理 at 上海复旦张江生物医药股份有限公司30年制药企业生产运营管理经验。先后在外资制药企业、民营制药企业及上市制药企业担任生产经理、集团副总经理及子公司总经理。
多次到欧、美、日等国家学习制药行业生产运营管理。对制药行业现代管理有深刻理解和丰富的实践经验。荣获上海市实施发明成果优秀企业家,区拔尖人才。
CGT产品GMP审计中发现的问题与分析 Analysis of defects found in GMP audits of CGT products
Speakers

董立玮
亚太区首席合规顾问Principal Consultant APAC Compliance at 精鼎医药,Parexel International董立玮在制药行业拥有超过15年从业经验,精通药品从研发到临床批次生产直至商业化全生命周期的法规符合,尤其关注中、美、欧制药法规框架,擅长质量体系建立与改进、官方检查和监管行动准备与应对、数据可靠性整改与提升、审计与有因调查等。2018年加入精鼎医药任战略合规咨询首席顾问,为全球制药和生物制药客户提供GMP相关咨询,包括协助客户通过各国官方检查、执行EU QP审计、执行或协助应对授权许可(License in/out)项目的合规尽职调查、提供培训等。在加入精鼎之前,立玮在诺华制药担任合规经理。立玮毕业于中国药科大学国家生命科学与技术人才培养基地,获生物工程学士学位。 Liwei Dong has over 15 years of experiences in the pharmaceutical industry. He is proficient in regulatory compliance of pharmaceutical products lifecycle from R&D to manufacturing of clinical batches till commercialized manufacturing, especially in the regulation framework of China, US, and EU. He excels at establishing and maintaining quality systems, preparing and responding Health Authority legal enforcements, remediating and improving data integrity, auditing and for-cause investigating etc. He joined Parexel in 2018 as a Principal Consultant in Strategic Compliance Consulting, and provides GMP consultation to pharmaceutical and biotech customers worldwide, including to support clients to pass various inspections, to conduct EU QP audits, to execute or defend due diligence audits in license in/out projects, and to provide trainings etc. Prior to join Parexel, Liwei worked as Compliance Manager in Novartis. Liwei has a bachelor’s degree on bioengineering at China Pharmaceutical University in the National Base of Life Science and Biotechnology Education.
Operational Readiness
Speakers

John Vaughn
Vice President, Asia of CAIJohn serves as the Vice President, Asia Life Sciences for CAI. With progressive responsibility within the organization over the past ten years, he has served as project manager, client manager, area client manager, sales manager, and regional director, including four years in Asia Pacific. Over his 20-year career, John’s technical background is in process calibration and metrology, validation, and computerized asset management systems. With previous roles as calibration manager and C&Q manager and extensive field experience in CQV along with ISA certifications in instrumentation and controls, he brings a strong level of technical competence to leadership roles.
全球三大洲符合ISO标准的培养基生产基地确认供应链安全
Speakers

林森
上游工艺解决方案技术总监 at 默克新版欧盟GMP无菌附录的主要变化及无菌保障能力提升策略 Major Changes in EU GMP Annex 1 Manufacture of Sterile Medicinal Products and Strategies for Enhancing Sterile Assurance Capabilities
Speakers

张新
顾问教授Consultant professor at 沈阳药科大学Shenyang Pharmaceutical University张新女士,沈阳药科大学顾问教授,制药工程正高级工程师,质量工程师,六西格玛黑带。曾在国内多家知名制药企业担任质量负责人,质量受权人和工厂运营负责人;擅长质量体系搭建和推动质量管理水平提升,多次接受国内及FDA的无菌产品现场检查,积累了丰富的实战经验。多次赴美欧参加ISPE、PDA等组织的质量管理培训和学术研讨会议;多次应邀赴东南亚、中东国家讲解ICHQ10及GMP相关指南;作为第一副主编,出版高校教材《药品生产质量管理规范》(案例版);作为工业界代表参与了国家药监局审核查验中心组织的《ICH Q9质量风险管理(R1)》工作组工作及新版GMP指南的修订工作。Ms. Zhang Xin is a consultant professor at Shenyang Pharmaceutical University. She is a professorate senior engineer in pharmaceutical engineering, a quality engineer, and a six sigma black belt. She has served as the head of quality, the qualified person and the head of operation in many famous pharmaceutical companies in China. She is an expert in building quality systems and promoting the improvement of quality management. She has hosted numerous regulatory on-site inspections for sterile products by both NMPA and FDA, and has accumulated rich practical experience. She has been to the United States and Europe for multiple times to participate in quality management training and academic seminars organized by ISPE, PDA, etc. She has been invited to the Southeast Asia and the Middle East countries for multiple times to give lectures on ICHQ10 and GMP related guidelines. As the first deputy editor-in-chief, she has published the textbook for colleges and universities, Good Manufacturing Practice (Case Studies). she has taken part in the work of the working group of ICH Q9 Quality Risk Management (R1) and the revision of the new version of the GMP Guideline organized by CFDI as a representative of the industry.
细胞治疗/溶瘤病毒/AAV产品药学变更的CMC考虑 CMC considerations for pharmaceutical changes in cell therapy/oncolytic virus /AAV products
Speakers

曹晓平 Ph.D.
高级副总裁、技术运营部负责人 at 上海药明巨诺生物科技有限公司曹晓平博士拥有超过21年在美国、中国和亚太地区的全球生物制药研发经验,成功支持了多款创新药物与疫苗在中国和美国的研发与上市,专长与经验包括新药开发、药学研究、生产、注册、及供应链管理等。
曹博士现任药明巨诺高级副总裁与技术运营部负责人,负责细胞治疗产品的工艺开发、技术转移、临床与商业生产、供应链及工程设施设备等。
加入药明巨诺之前,曹博士为辉瑞公司高级总监和全球药学中国负责人,组建并带领团队支持了辉瑞在中国的化学药、生物制剂及疫苗产品的药学注册申报,推动多个产品在华获批并支持产品生命周期管理。在此之前,曹博士曾在辉瑞美国和中国担任多个技术与管理职务,在制剂与工艺、药物材料科学、及分析技术方面积累了丰富经验与专长。
曹博士还曾担任默沙东中国研发药学负责人,组建并带领团队为临床试验申请、新药上市申请及上市后的药品提供药学支持,成功支持了多款关键药物与疫苗在中国的临床与上市批准。此外,曹博士还曾在位于新加坡的一家专注脑健康的创新药公司Cerecin担任副总裁、负责公司产品的药学研究、临床及商业生产与供应。
曹博士拥有威斯康星大学麦迪逊化学博士学位、康涅狄格大学MBA学位及清华大学化学学士学位。
CGT的全球临床供应实践 Global Clinical Supply Practices for CGT
Speakers

施维维
中国质量负责人 Head of Quality, at 赛默飞临床试验事业部Thermo Fisher Clinical Trail Division ChinaVela于2014年加入赛默飞Patheon制药服务事业部临床试验服务苏州工厂,目前担任临床试验服务中国区质量负责人,主要负责维护并改进赛默飞临床试验服务中国区的质量管理体系,从而确保临床药品的持续供应和妥善管控,使其符合客户的预期用途、满足客户的规格要求和质量标准。Vela具有药学专业背景,同时因有丰富的质量管理合规经验,拥有15年以上医药质量管理相关工作经验 。
临床试验供应链的仿真及关键决策优化/Clinical Supplies Simulation and Strategic Decision Optimization
Speakers

付迪宇Orville
创始人 战略及业务发展负责人Founder and Head of Strategy and Business Development at 华升智药科技开发(北京)有限公司 Huasheng Pharmatech (Beijing) Co., Ltd.付迪宇 拥有超过13年的跨国医药和生物科技企业创新药临床试验供应链管理经验。他曾带领团队负责多个全球及区域临床试验供应链的策略设计,并成功实施,支持新药在欧洲、美国、亚太等多个重要国家和地区上市,展现了他卓越的领导能力和深厚的专业知识。作为曾在百济神州生物科技的临床供应管理副总监和辉瑞临床试验供应的高级经理,付先生在行业内建立了显著的成就。付先生于2021年北京大学光华管理学院攻读MBA,师从管理科学与信息系统系彭一杰教授,这一经历不仅为他提供了坚实的供应链仿真优化理论基础,也激发了他在该领域的创新思维和战略规划能力。
近期,他和几位跨行业领导者联合创立华升智药科技开发(北京)有限公司 目前担任战略及业务发展负责人。华升智药是一家深度科技公司,通过使用基于蒙特卡洛模拟、贝叶斯算法等技术开发的仿真模拟平台及专业技术服务,为各类制药公司、生物科技公司、研发合同性组织(CRO)提供专业的供应链咨询服务及全链条优化的解决方案。
Orville Fu has over 13 years of experience in clinical trial supply chain management for innovative drugs within multinational pharmaceutical and biotech companies. He has led teams in strategy development and successful implementation of global and regional clinical trial supply chains, until the approval of new drugs in key countries and regions including Europe, the United States, and Asia-Pacific, demonstrating his outstanding leadership abilities and deep professional knowledge. As the former Associate Director of Global Supply Chain at BeiGene and Senior Manager of Clinical Trial Supply at Pfizer, Mr. Fu has established significant achievements in the industry. Mr. Fu pursued an MBA at Guanghua School of Management, Peking University in 2021, under Professor Peng Yijie of the Department of Management Science and Information Systems. This experience not only provided him with a solid foundation in supply chain simulation optimization theory but also inspired innovative thinking and strategic planning capabilities in the field.
Recently, with several leaders from the industry, he co-founded Huasheng Pharmatech (Beijing) Co., Ltd., where he currently serves as the Head of Strategy and Business Development. Huasheng Pharmatech is a deep-tech company that offers professional supply chain consulting services and comprehensive optimization solutions to pharmaceutical companies, biotech companies, and Contract Research Organizations (CROs) through a simulation platform and professional technical services developed vial technologies like Monte Carlo simulation and Bayesian algorithms.

茅亚超Robin
联合创始人 产品研发负责人Co-Founder and Head of Product Development at 华升智药科技开发(北京)有限公司Huasheng Pharmatech (Beijing) Co., Ltd.茅亚超,华升智药科技开发(北京)有限公司的联合创始人、产品研发负责人,拥有丰富的工业互联网、ToB SAAS软件服务行业、百亿级日业务数据处理系统的架构、产品商业化经验。在加入华升智药之前,曾任慧策集团核心产品线负责人,其带领下的产品团队为ERP数字化领域带来了全渠道的业务解决方案。
作为CISAW(信息安全保障人员认证)的高级成员,他在工业互联网开发信息安全方面展现出了深入的专业知识。他同时也是国际制药工程协会(ISPE)的一员。茅亚超先生在北京大学光华管理学院 获得MBA学位,同时拥有计算机科学与技术专业的工学硕士学位。无论是在技术革新、产品开发还是团队管理方面,茅先生都展现出了他的专长和对卓越的不懈追求.
Robin Mao, co-founder and head of product development at Huasheng Pharmatech (Beijing) Co., Ltd., has extensive experience in industrial Internet, ToB SAAS software services, and the architecture and commercialization of systems handling daily business data on the scale of billions. Before joining Huasheng Pharmatech (Beijing) Co., Ltd, he was a key product line manager at HuiCe Group, leading product teams to introduce omnichannel business solutions in the ERP digitalization field. As a senior member of CISAW (Information Security Assurance Certification), he has demonstrated deep professional knowledge in information security within industrial Internet development. He is also a member of the International Society for Pharmaceutical Engineering (ISPE). Mr. Mao earned his MBA from Peking University's Guanghua School of Management and holds a Master of Engineering degree in Computer Science and Technology. Whether in technological innovation, product development, or team leadership and management, Mr. Mao has shown his expertise and relentless pursuit of excellence.
ISPE无菌药品生产设施指南解读 Baseline Guide Vol Vol 3 introduction: Sterile Product Manufacturing Facilities 3rd Edition
Speakers

Aaron Weinstein
Senior Director, Compliance Consulting of IPS – Integrated Project Services, LLC对FDA bioassay方法建立指南的考虑 Consideration of FDA guidelines for the establishment of bioassay methods
Speakers

刘雅容,Ph.D
CEO of 沙砾生物刘雅容, 美国南加州大学博士、博士后,沙砾生物创始人、首席执行官,中山大学孙逸仙纪念医院逸仙医学客座教授;江苏省双创人才、姑苏领军人才、苏州工业园区科技领军人才、上海市海外高层次人才专家、上海市浦江人才;正高级研究员,CSCO会员,女医师协会会员,浦东新区青年联合会委员;拥有10余年从事腺病毒、慢病毒等各种病毒载体用于定向基因治疗的研究经验,以及慢病毒产业化的经验;在Nature Communications, Science Immunology,Gene Therapy,Molecular Therapy 等国际顶级杂志发表40多篇论文。并拥有1项美国技术专利,申请细胞治疗领域发明专利90余项,主持上海市生物医药科技支撑项目1项,作为第二完成人获得2017年浦东新区科技进步奖创业团队三等奖;2022年浦东新区青年优秀企业家、2021年获得上海市创业新秀、长三角女性创新创业大赛银奖。2019年创立沙砾生物,致力于创新细胞疗法TIL在实体瘤的研发和临床应用。
Yarong Liu, Ph.D., postdoctoral fellow, University of Southern California, founder and CEO of Grit biotechnology, Visiting Professor of Yixian Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University; Double Innovation talents of Jiangsu Province, leading talents of Gusu, leading talents of Science and Technology of Suzhou Industrial Park, experts of overseas high-level talents of Shanghai, talents of Shanghai Pujiang; Senior researcher, Member of CSCO, member of Women's Medical Association, member of Pudong New Area Youth Federation; She has more than 10 years of experience in the research of various viral vectors such as adenovirus and lentivirus for targeted gene therapy, and experience in the industrialization of lentivirus. She has published more than 40 papers in top international journals such as Nature Communications, Science Immunology, Gene Therapy, Molecular Therapy, etc. She has one American technology patent, applied for more than 90 invention patents in the field of cell therapy, presided over one project of Shanghai biomedical science and technology support, and won the third prize of the entrepreneurship team of Pudong New Area Science and Technology Progress Award in 2017 as the second complete person; In 2022, Pudong New Area Young Outstanding Entrepreneurs, and in 2021, won the Silver Award of Shanghai Start-up Rookie and Yangtze River Delta Women Innovation and Entrepreneurship Competition. In 2019, She found Grit biotechnology, which is committed to the development and clinical application of innovative cell therapy TIL in solid tumors.
隔离器的灭菌与验证 Sterilization and validation for Isolator
Speakers

柯桂盛
制剂工艺经理 Production Process Manager at 深圳市海普瑞药业集团股份有限公司Shenzhen Hepalink Pharmaceutical Group Co., Ltd.柯桂盛: 2018年加入深圳市海普瑞药业集团股份有限公司 现任制剂工艺经理,主要负责工艺转移、工艺优化、变更及验证等工作。参与多条液体制剂线建设,项目通过中国、欧盟、美国的GMP认证;参与了“药品GMP指南(第2版)无菌制剂分册”编写工作。 Ke Guisheng: Joined Shenzhen Hepalink Pharmaceutical Group Co., Ltd. in 2018. Currently serving as the Manager of Production Process, mainly responsible for process transfer, process optimization, changes, and validation. Participated in the construction of multiple liquid formulation lines. Projects have passed GMP certification in China, the European Union, and the United States. Also participated in the compilation of the "Drug GMP Guidelines (2nd Edition) Aseptic Formulation Volume."
针对细胞和基因治疗的脱靶和随机插入的分析 Analysis of off-target and random insertions for cell and gene therapy
Speakers

谭炳合,Ph.D
副总裁 at 邦耀生物谭炳合博士现为上海邦耀生物科技有限公司CMC副总裁,分管公司管线产品的CMC和生产制备工作。在细胞与基因治疗产品的开发和临床应用上积累多年实践经验,擅长使用新型基因编辑技术开发通用型细胞治疗技术&产品。以第一作者和共同作者身份在Nature、Advanced Science 、Cancer Research、Cancer Immunology Research等著名期刊上发表多篇研究论文。申请国际发明专利2项、中国发明专利4项,主持1项国家自然科学基金青年项目,作为技术骨干参与多项国家自然科学基金项目。目前正在负责多项CGT创新药的开发、制备、检验和注册申报工作,带领团队成功将三款细胞和基因治疗产品推进临床研究阶段。 Dr. Tan Binghe is currently the vice president of Shanghai BRL Medicine Co., Ltd., in charge of CMC and production of the company's pipeline products. He has accumulated many years of practical experience in the development and clinical application of cell and gene therapies, and is proficient in gene editing to develop advanced allogeneic-universal cell products. As the first author and co-author, he has published research papers in famous journals such as Nature, Advanced Science, Cancer Research and Cancer Immunology Research. He applied for 2 international invention patents, 4 invention patents in China, presided over a youth project of the National Natural Science Foundation, and participated in several projects of the National Natural Science Foundation as the key technician. At present, he is responsible for the development, preparation, inspection and registration of a series of CGT innovative therapies, and has successfully applied for IND approval for three CGT therapeutic products in China.
总结,会议结束 Summary , Meeting Adjourned
Speakers

王麟Lynn
Head of Project and Alliance at 药明合联 WuXi XDC Co. Ltd总结,会议结束 Summary , Meeting Adjourned
Speakers

高杨 博士
首席战略官(CSO) at 上海邦耀生物科技有限公司高杨博士目前担任上海邦耀生物科技有限公司的首席战略官,全面负责公司基因和细胞治疗产品的开发、申报、项目资产管理等工作,协助CEO整个公司的经营
加入上海邦耀生物科技有限公司之前,高博士在制药行业拥有近20年的管理和开发经验,先后在国家药品监管机构、跨国制药企业、生物科技公司和全球CRO公司担任技术/管理工作,熟悉制药行业全链条的运转之道,尤其在药物开发策略、申报策略、质量体系建设和商业化战略方面有着全面、丰富的经验。曾是中国国家药品监督管理局药品审评中心的高级审评官,8年时间内审评过千份申报项目并执行过几十次现场检查,涵盖跨适应症领域的新药临床申请、新药上市申请、仿制药申请、上市后补充申请等技术审评工作,对监管科学和CDE、FDA、EMA及ICH的技术指南有着全面深刻的理解。之后4年担任礼来公司的法规CMC和医疗器械负责人,领导多个创新药CMC策略、技术转移策略和医疗器械注册策略的制定和执行,参与礼来全球加快研发的改革工作,并为礼来在华合作伙伴的创新药研发和商业化提供技术支持。同期兼任RDPAC的CMC委员会轮值主席,领导7个工作组为2015年启动的中国药监改革积极建言献策,为构建支持药物创新的新生态系统做出了杰出贡献。曾担任苏州亚盛制药的法规事务总监,制定并执行中美研发和注册策略,成功获批中、美多个IND。之后担任PAREXEL的首席药物研发和法规顾问,3年时间内为多达30家国内外生物制药公司约50个项目提供研发和监管策略的咨询服务,成功助力他们在中美获得监管部门的批准并开展新药研发。业余时间,高博士积极探索和实践人工智能和数字技术赋能药物研发,设计并开发远程智能化临床试验平台。
总结,会议结束 Summary , Meeting Adjourned
Speakers

甘益民
集团副总经理/子公司总经理 at 上海复旦张江生物医药股份有限公司30年制药企业生产运营管理经验。先后在外资制药企业、民营制药企业及上市制药企业担任生产经理、集团副总经理及子公司总经理。
多次到欧、美、日等国家学习制药行业生产运营管理。对制药行业现代管理有深刻理解和丰富的实践经验。荣获上海市实施发明成果优秀企业家,区拔尖人才。
ISPE CEO致欢迎辞 (Welcome Speech by ISPE CEO )
Speakers

Thomas B. Hartman
President and CEO of ISPEFDA中国办公室官员演讲 (FDA China Official Speech) FDA‘s view point on advanced pharmaceuticals manufacturing
Speakers

Tonia Bernard
Assistant Country Director助理主任 of FDA China OfficeFDA中国办公室Tonia Bernard is an Assistant Country Director with the FDA’s OGPS China office, where she performs inspections of pharmaceutical facilities located in China. Prior to joining the FDA China Office, Ms. Bernard has more than 5 years of field experience with FDA’s ORA performing a broad range of domestic and foreign drug inspections. She earned a Master of Public Health in Environmental Science from Fort Valley State University in Fort Valley, Georgia and a Bachelor of Science in Exercise Science from University of Maryland Eastern Shore in Princess Anne, Maryland.
贝妮娜,公共卫生硕士
FDA中国办公室助理主任
贝妮娜在FDA中国办公室任助理主任,开展对中国制药企业的现场检查工作。在加入中国办公室之前,贝妮娜在FDA监管事务办公室(ORA)有超过5年的工作经验,在国内国外进行广泛的药品检查。她在乔治亚州福特谷的福特谷州立大学获得环境科学公共卫生硕士学位,并在马里兰东岸大学获得运动科学学士学位。
会议主席致欢迎辞 Welcome Speech by Chair
Speakers

李锦才博士
CEO of 药明合联会议主席开场致辞及分委会介绍 Opening speech by Chair
Speakers

王卫兵 Alex
副总裁VP at 江苏恩华药业,Jiangsu Nhwa Pharmaceutical Co.,Ltd会议主席开场致辞 Opening speech by Chair
Speakers

王卫兵 Alex
副总裁VP at 江苏恩华药业,Jiangsu Nhwa Pharmaceutical Co.,Ltd会议主席开场致辞及分委会介绍 Opening speech by Chair
Speakers

康伟
副总裁VP at 奥星集团 Austar Group0+ years of ISPE member Member of ISPE China Biological Committee Vice President, Austar, 2019-01 to Present Senior specialist and SME, NNE 2007- 2019
With 28+ years of work experience in biopharmaceutical and pharmaceutical industry as project sponsor and project lead, Mr. Kang Wei takes role as Vice President in AUSTAR Group at present and focus on pharmaceutical engineering and project management. He works on strategy planning and biotech facility engineering as international biotech facility expert with visionary approach. Highlight clients & projects (GMP commercial production facility) as follows: Bill & Melinda Gates Foundation, NovoNordisk, GSK, Novozymes, Boehringer-Ingelheim, Adimmune, Henlius, Wuxi Biologics, Junshi, BeiGene, Carsgen, Gracell.
Mr. Kang Wei coordinated with international team to provide professional consulting services for international and domestic clients in terms of Vaccine, Monoclonal Antibody, ATMP, ADC, etc., the project standards comply with FDA, EMA, WHO and NMPA.
International organizations and forums:
“Establishing Biotech Manufacturing Capacity in China” in 2015, IBC Life Sciences D development and Production (BDP) Week, in Huntington Beach, CA.
“Bio facility consideration from engineering perspective” in 2016, ISPE Biologics Development Symposium in Hangzhou
“Best practice for Bio project execution “in 2017, ISPE Biologics Forum in Benxi
“Global Practice Bridge to Local Manufactures “in 2018, ISPE / CIPM in Chongqing
“THE MANUFACTURING ROAD “Panel discussion in 2019, BioCentury & BayHelix China Summit in Shanghai
“GMP trend and expectation for cell therapy facility” in 2019, ISPE "Innovative technology and concept for state-of-the-art biologic facility" session in Changsha
“Case study - Compliant and Efficient biologic facility “in 2020, ISPE / CIPM in Chongqing
会议主席开场致辞及分委会介绍 Opening speech by Chair
Speakers

孙京林
副总裁VP at 中国生物技术股份有限公司, China National Biotec Group Company Limited (CNBG)A PI :良好 实 践 指 南 :运 营 管 理 ( I SP E指 南 ),与I CH Q 1 1 整合 API: Good Practice Guide: Operations Management (ISPE guideline), intergrate with ICH Q11
Speakers

周臻弘
质量副总裁 at 浙江海正药业Vice President of Quality at Zhejiang Hisun Pharmaceutical Co., Ltd., has worked in multiple departments including production, R&D, sales, and quality, especially in quality department for over 20 years. He has served as the editorial board member for the Quality Management System sub-volume and API sub-volume of the Chinese GMP Guidelines (2nd edition). 目前担任浙江海正药业质量副总裁,历经生产,研发,销售,质量等多个部门,在质量部门工作超过20年,担任中国药品 GMP指南(第2版)质量管理体系分册和原料药分册的编委工作。
基于CCS的环境监控解决方案 CCS-based Environmental Monitoring Solution
Speakers

徐敏凤 Minfeng XU
产品总监Chief Product Officer at 麦克微尔(上海)科技有限公司 Micronview麦克微尔(上海)科技有限公司环境监测全系列产品总监,包括监测仪器和数字化、智能化解决方案等。徐敏凤近30多年均从事无菌医药生产管理和医药行业合规管理工作,是洁净室(区)和相关设施验证专家,环境监控及风险控制专家。洁净检测国家标准GB/T 16292-16294《医药工业洁净室(区)悬浮粒子、浮游菌和沉降菌的测试方法》项目负责人和主要起草人,翻译出版了有关专著《Thermal Validation in Moist Heat Sterilization》(湿热灭菌工艺的验证)和《Aseptic and Sterile Processing Control, Compliance and Future Trends》(无菌与灭菌-工艺控制与合规及未来趋势)等。
生物制品MAH制度 implementation of the MAH system for biological products
Speakers

潘海龙
质量管理部主任 at 中国生物技术股份有限公司生物制品质量管理、生产管理、质量控制
Quality management production management and quality control for biological products
2002.09~2008.09,成都生物制品研究所质量保证部经理助理
Quality Assurance Department Assistant of Chengdu Institute of Biological Products Co.,Ltd.
2008.09~2017.09,成都生物制品研究所质量检定室主任
Quality Control Department Manager of Chengdu Institute
2017.09~2019.08,成都生物制品研究所细菌性疫苗室主任
Bacterial Vaccine Department Manager of Chengdu Institute
2019.08~2020.05,成都生物制品研究所生产管理部经理
Production Management Department Manager of Chengdu Institute
2020.5~至今 中国生物技术股份有限公司质量管理部主任
2020.5 till now, Quality Management Department Director of China National Biotec Group Co., Ltd..
隔离器无菌生产的挑战 Challenges with Aseptic Production in An Isolator
Speakers

顾晨
资深产品经理Senior Product Management at 星德科包装技术(杭州)有限公司Syntegon Packaging Technology (Hangzhou) Co., Ltd._工程学硕士学位,12年液体制剂产品相关经验,主要关注隔离器技术产品的开发。
Engineering Master Degree. 12 year pharma liquid machine experience, focus on Isolator technology product development.
ISPE GEP指南介绍 ISPE GEP Guideline Introduction
Speakers

Chip Bennett
Associate Director, Global C&Q of CAIA Project Manager and Senior Validation Engineer, Chip is a PMI® Certified Project Management Professional (PMP), a recognized industry expert, speaker, and published author, and a consultant with over 20 years of experience in the pharmaceutical and regulated non-pharmaceutical industries and with expertise in risk-based verification, aseptic manufacturing, cleaning validation, quality systems, and owner project management. Chip is responsible for developing and implementing Quality Risk Management (QRM) based Commissioning and Qualification programs and projects, with a focus on assessing and training clients regarding development, implementation, and transition to risk-based approaches. Chip was a lead author on the ISPE Good Practice Guide: Good Engineering Practice, 2nd Edition (2021).
CCS :污 染 控 制 策 略/附 件 1 CCS: Contamination Control Strategy/Annex 1
Speakers

徐禾丰
行业专业人士 Industry professional徐禾丰,从1984年开始从事制药行业工作,先后从事过生产车间工作、国际贸易工作、注册与质量管理工 作,咨询工作,现在已经退休。先后编写多个FDA、EDMF、COS/CEP等注册文件;翻译了大量的FDA、EU、ICH、WHO、PIC/S、PDA等机构和组织相关法规、指南;翻译出版了许多GMP法规相关的书籍,发表了大量的GMP法规相关论文。先后为各地药监局GMP检查员做了多次培训。
Xu Hefeng began to work in the pharmaceutical industry in 1984, and has been engaged in production workshop work, international trade work, registration and quality management work, consulting work, and is now retired. Has written several FDA, EDMF, COS/CEP and other registration documents; Translated a large number of FDA, EU, ICH, WHO, PIC/S, PDA and other agencies and organizations related regulations and guidelines; He has translated and published many books related to GMP regulations, and published a large number of papers related to GMP regulations. Has done many training for the GMP inspectors of the local drug administration.
ISPE指南介绍:APQ:PPPQMS Introduction for APQ Guide: Process Performance & Product Quality Monitoring System (PPPQMS)
Speakers

Dr. Rainer Nicolai
Product Owner Engineering Consulting of F. Hoffmann - La Roche AGDr. Rainer Nicolai is the product owner Engineering Consulting at Roche’s headquarter site at Basel/Switzerland. He has worked as a project manager for more than 20 years and is a subject matter expert on containment technologies covering the whole manufacturing value chain.
浅析无菌操作的发展趋势(operation trend in aseptic production )
Speakers

娄再飞
验证与合规负责人 at 星德科包装技术(杭州)有限公司娄再飞 :星德科包装技术(杭州)有限公司 现任验证与合规部门负责人,2011年加入星德科,主要负责制药包装设备的技术文档,验证和法规符合性等工作。曾在国内外知名药企从事药品研发,无菌药品生产验证和法规符合性工作,拥有近20无菌药品生产经验。
Lou Zaifei: Department head of service for validation and compliance in Syntegon Packaging Technology (Hangzhou) Co., Ltd.,. Joined Syntegon in the year of 2011, is mainly responsible for delivering of technical documentation, validation and regulatory compliance of pharmaceutical equipment. Previously worked in famous pharmaceutical company for drug R& D, sterile drug production&validation and compliance. With about 20 years of experience in aseptic process.
良好规范指南:有 效 化 合 物 的 防 护 +共 用 设 施 Good Practice Guide: Containmen t for Pot ent Compounds+ Shared facilities
Speakers

夏禄华
副总经理 at 江苏金迪克生物科技有限公司夏禄华,现任职于江苏金迪克生物科技有限公司副总经理,主要从事质量管理工作,先后在海正药业、贝朗医疗、药明生物,康希诺生物工作,负责药品生产质量管理工作。作为工业界代表参与了国家药监局组织的《药品共线生产质量管理指南》,《临床试验用药品生产质量管理规范》,《ICHQ9质量风险管理(R1)》,《抗体类药物现场检查指南》,药品GMP指南-无菌制剂分册等指南的起草和修订。国家药监局高级研究学院特聘专家,浙江省药品审评核验中心特聘专家,上海市药监局特聘专家。
高活化合物的处理和操作 Handling and Operation of potent compounds
Speakers

朱人
Golder IH业务负责人 Associate Director, Environment of 科进公司WSPWilliam Zhu is a Certified Industrial Hygienist (CIH) and Associate Director of WSP Environment China. He has extensive experience in potent compound industrial hygiene/ occupational health risk management including risk assessment, containment design, containment performance assessment. He provides consulting services to multinational and local companies in the pharmaceutical industry. He has a bachelor's degree in Preventive Medicine from Fudan University in 2002 and a master's degree in occupational health in 2005. 朱人是一名美国注册工业卫生师(CIH),科进柏诚环境服务上海办公室副技术总监。他在活性药物成分的工业卫生/职业健康风险管理方面具有丰富的经验,包括风险评估、密闭设计、密闭性能验证等。他为制药行业的国际和国内公司提供咨询服务。他2002年获得复旦大学预防医学学士,2005年获得劳动卫生学硕士。
新版欧盟附录1的主要变化及湿热灭菌方面的实践解读 key highlight of revised Annex I of EU GMP and example sharing in moist heat sterilization
Speakers

胡仲新 Jessica
资深质量总监Sr. Director, Quality of 百特上海医疗仪器有限公司Baxter Healthcare胡仲新女士 1995 年毕业于中国药科大学,先后在武田制药,中美史克,北京诺华制药,罗氏诊
断和百特中国投资从事质量控制和质量管理工作,熟悉欧盟 GMP/GDP,世界卫生组织
GMP/GDP,澳大利亚 GMP,ICH/PICS 指南,韩国 GMP,台湾 GMP/GDP 及中国 GxP 等药品相
关法规要求,ISO 13485,中国医疗器械 GMP 及相关附录, 21CFR part 820, MDR, IVDR 等医疗
器械相关法规要求。在过往 23 年药品行业和 5 年医疗器械行业质量管理过程中,多次带领团队
顺利通过欧盟 GMP(包括无菌产品和片剂产品),世界卫生组织 GMP,澳大利亚 GMP 认证。
产品出口世界超过 160 个以上的国家。并参与了超过 5 个跨国药品技术转移项目和 70 个诊断试
剂的转移项目的质量保证工作,积累了丰富的药品/医疗器械生产的质量监管,委托生产监管,供
应商质量管理,研发和临床及注册的质量监管,上市后药物警戒的质量监管等经验。
Jessica Hu was graduated from China Pharmaceutical University in 1995. She has been worked
in Takeda, GSK, Beijing Novartis Pharama, Roche Dia and Baxter China, mainly in quality
control and quality assurance area. She is very familiar with worldwide GMP/GDP
requirements, including EU GMP/GDP(sterile products and tablet products), WHO
GMP/GDP, Australia GMP, ICH/PIC/S guideline, Korea GMP, Taiwan GMP/GDP and China GxP
related drug regulation requirements, as well as, ISO 13485, China MD GMP, US 21CFR part
820, MDR, IVDR related medical device regulation requirements. Lead the team successfully pass MHRA, WHO, TGA GMP inspection without major findings multiple times in past 21 years
working experiences in Pharma and 5 years working experiences in medical device area. Act
as quality workstream lead, successfully transferred 5 cross region drug products and more
than 70 IVD products. Have rich experiences in manufacturing supervision, CMO supervision,
supplier management, quality supervision in R&D, clinical trial, RA and PMS area etc.
企业解决方案高质量、低风险,快速灵活的赋能您的生物制药产能建设 Enterprise Solutions enabling your biomanufacturing capacity with high quality, low risk and high speed and flexibility
Speakers

何永江
Senior Project Manager, Enterprise Solutions企业解决方案高级项目经理 at Cytiva何永江毕业于天津大学,有着14年以上的制药工程行业工作经验, 并取得过PMP/6σ黑带及CWI等国际认
证。曾就职于颇尔过滤器有限公司先后担任过工艺工程师及项目经理。现作为Cytiva企业解决方案高级项
目经理任职于Cytiva企业解决方案部门,拥有着丰富的相关工艺知识和行业项目管理经验并成功管理参与
过多个客户企业解决方案项目。
Yongjiang He gratitude from Tianjin University, have more than 14 years of experience and is
PMP/ Six Sigma BB/ CWI certified. He has worked as a process engineer and project manager in
Pall Now as a senior project manager of Cytiva Enterprise Solutions, he is working in Cytiva
Enterprise Solutions department, with rich process knowledge and industry project
management experience and has successfully managed and participated in many customer
enterprise solution projects.
生物制药厂房设计与实施:策略优化与案例分析 Design and Implementation of Biopharmaceutical Manufacturing Facilities: Strategy Optimization and Case Study
Speakers

Mark Stephens
SME at Exyte (Shanghai) Co., Ltd.Mark,Exyte生物制药&生命科学课题专家,具有丰富的生物制药设施项目经验,主导过澳洲、欧洲、中国等多地国际知名项目,为诺华、默克、辉瑞等企业提供服务,致力于为生命科学领域的发展贡献力量。
Mark is a seasoned professional in the Biopharma & Life Sciences sector, with extensive expertise in designing and constructing biopharmaceutical facilities. He has a proven track record of effectively overseeing prominent projects in Australia, Europe, and China, and has established collaborative partnerships with industry leaders such as Novartis, Merck, Pfizer, and other key players. Mark's unwavering commitment to excellence, combined with his profound knowledge, drives his mission to lead the industry forward and foster continuous progress.
企业对高活化合物的综合管理 Management of highly potent compounds by pharmaceutical companies
Speakers

罗建军J.J. Luo
生物偶联产品研发和生产副总裁VP of Bioconjugate Product Development and Manufacturing at 无锡药明合联生物技术有限公司WuXi XDC Co. Ltd生命周期管理:APQ指南:过程 性 能 和 产 品 质 量 监 控 系 统 ( PPPQ M S) (ISPE 指南) Lifecycle Management : APQ Guide: Process Performance & Pro duct Qual ity Monitori ng System (PPPQMS) (ISPE guid eline)
Speakers

Mr. Maurice B. Parlane , B Tech MIT
Principal/Director of New Wayz Consulting Ltd/CBE Pty LtdMaurice Parlane is Principal of New Wayz Consulting Ltd in New Zealand and a Director of CBE Pty Ltd in Australia. He is a professional engineer with 30 years’ experience within the biopharmaceutical industry, including 20 years as an industry consultant providing support to manufacturing and compliance management; validation and operational excellence projects in Australasia and the Asia Pacific region. Prior to this, he held senior engineering and manufacturing roles within the Glaxo group of companies. He has a Bachelor of Manufacturing Technology (Hons) as well as mechanical and electrical engineering qualifications. Maurice is past president and current director of the ISPE Australasian Affiliate. He is the co-lead of ISPE’s Asia Pacific Regulatory and Quality Harmonization committee and leader of the Process Validation Team and is a member of the Guidance Documents Committee. He is an ISPE PV Instructor and was named ISPE Member of the year in 2016. He is currently serving as the Leader of the ISPE Process Validation Team.
与国际接轨-中国版《无菌药品生产污染控制策略(CCS)技术指南》介绍
Speakers

肖志坚
质量负责人 at 百济神州中国南京大学生物化学系毕业,中欧全球EMBA,现任百济神州中国质量负责人,历任诺华亚太、中东、非洲和日本区域国家质量部负责人,阿斯利康亚太地区质量负责人等。熟悉全球质量体系,有丰富的跨国团队领导的经验
ISPE指南:基于风险的药品生产第2版 ISPE Guidance: Risk-Based Manufacture of Pharma Products 2nd Editio 视频
Speakers
Ms. Stephanie A. Wilkins, PE
President of PharmaConsult US IncStephanie Wilkins, PE, Lean Six Sigma Green Belt, has over 30 years of professional experience in project management, risk management, engineering and validation solutions for the pharmaceutical and biotech industry including research, development, and pilot plant and manufacturing facilities. She is president of PharmaConsult US, Inc, which provides cross-contamination and containment consulting to the pharmaceutical industry. Wilkins, a member of ISPE since 1993 is the co-Chair of the ISPE Risk-MaPP Baseline Guide Task Team and was a member of the ISPE International Board of Directors. Wilkins is an ISPE trainer for Managing Cross Contamination and Risk-MaPP Principles. Wilkins also is a reviewer for articles to be placed in the Pharmaceutical Engineering Journal as well as contributed articles, given lectures and organized courses for ISPE. Wilkins graduated from the Pennsylvania State University with a Bachelor of Architectural Engineering.
血液制品生物工厂项目建设管理要点 Key points in the construction and management of plasma manufacturing projects
Speakers

郭维强
上海血制 副总经理 Vice General Manager at 天坛生物 Tiantan Bio国家一级建造师、医学生物工程师
First-class constructor/ Biomedical Engineer
主要项目经历:
成都蓉生永安血制项目(设计投浆1200吨,项目投资14.5亿) 项目经理
Chengdu Rongsen Yongan plasma facilities project (1200 ton/year
Plasma, investment 14.5 Billion) Projector
云南生物制品基地项目(设计投浆1200吨,项目投资16.5亿) 项目经理
Yunnan biological products facilities project (1200 ton/year
Plasma, investment 16.5 Billion) Projector
成都蓉生重组凝血因子项目(rhFVIII 26亿IU,项目投资5亿) 项目经理
Chengdu Rongsen recombinant FVIII facilities project (rhFVIII 26 Billion IU, investment 5 Billion) Projector
阿联酋Hayat疫苗工厂项目(项目投资3亿美金) 项目经理
UAE Hayat vaccinum facilities project (investment $16.5 Billion) Projector
主要擅长领域:
新建生物制品工厂项目全生命周期管理
Full Life Cycle Management of New Biological Products Factory Project
工厂预防性维护管理
Factory preventive maintenance management
设备设施验证
Equipment and facility validation
总结,会议结束 Summary , Meeting Adjourned
Speakers

康伟
副总裁VP at 奥星集团 Austar Group0+ years of ISPE member Member of ISPE China Biological Committee Vice President, Austar, 2019-01 to Present Senior specialist and SME, NNE 2007- 2019
With 28+ years of work experience in biopharmaceutical and pharmaceutical industry as project sponsor and project lead, Mr. Kang Wei takes role as Vice President in AUSTAR Group at present and focus on pharmaceutical engineering and project management. He works on strategy planning and biotech facility engineering as international biotech facility expert with visionary approach. Highlight clients & projects (GMP commercial production facility) as follows: Bill & Melinda Gates Foundation, NovoNordisk, GSK, Novozymes, Boehringer-Ingelheim, Adimmune, Henlius, Wuxi Biologics, Junshi, BeiGene, Carsgen, Gracell.
Mr. Kang Wei coordinated with international team to provide professional consulting services for international and domestic clients in terms of Vaccine, Monoclonal Antibody, ATMP, ADC, etc., the project standards comply with FDA, EMA, WHO and NMPA.
International organizations and forums:
“Establishing Biotech Manufacturing Capacity in China” in 2015, IBC Life Sciences D development and Production (BDP) Week, in Huntington Beach, CA.
“Bio facility consideration from engineering perspective” in 2016, ISPE Biologics Development Symposium in Hangzhou
“Best practice for Bio project execution “in 2017, ISPE Biologics Forum in Benxi
“Global Practice Bridge to Local Manufactures “in 2018, ISPE / CIPM in Chongqing
“THE MANUFACTURING ROAD “Panel discussion in 2019, BioCentury & BayHelix China Summit in Shanghai
“GMP trend and expectation for cell therapy facility” in 2019, ISPE "Innovative technology and concept for state-of-the-art biologic facility" session in Changsha
“Case study - Compliant and Efficient biologic facility “in 2020, ISPE / CIPM in Chongqing
基于风险的生产-理论与实践 Risk-based production - Theory and practice
Speakers

张新
顾问教授Consultant professor at 沈阳药科大学Shenyang Pharmaceutical University张新女士,沈阳药科大学顾问教授,制药工程正高级工程师,质量工程师,六西格玛黑带。曾在国内多家知名制药企业担任质量负责人,质量受权人和工厂运营负责人;擅长质量体系搭建和推动质量管理水平提升,多次接受国内及FDA的无菌产品现场检查,积累了丰富的实战经验。多次赴美欧参加ISPE、PDA等组织的质量管理培训和学术研讨会议;多次应邀赴东南亚、中东国家讲解ICHQ10及GMP相关指南;作为第一副主编,出版高校教材《药品生产质量管理规范》(案例版);作为工业界代表参与了国家药监局审核查验中心组织的《ICH Q9质量风险管理(R1)》工作组工作及新版GMP指南的修订工作。Ms. Zhang Xin is a consultant professor at Shenyang Pharmaceutical University. She is a professorate senior engineer in pharmaceutical engineering, a quality engineer, and a six sigma black belt. She has served as the head of quality, the qualified person and the head of operation in many famous pharmaceutical companies in China. She is an expert in building quality systems and promoting the improvement of quality management. She has hosted numerous regulatory on-site inspections for sterile products by both NMPA and FDA, and has accumulated rich practical experience. She has been to the United States and Europe for multiple times to participate in quality management training and academic seminars organized by ISPE, PDA, etc. She has been invited to the Southeast Asia and the Middle East countries for multiple times to give lectures on ICHQ10 and GMP related guidelines. As the first deputy editor-in-chief, she has published the textbook for colleges and universities, Good Manufacturing Practice (Case Studies). she has taken part in the work of the working group of ICH Q9 Quality Risk Management (R1) and the revision of the new version of the GMP Guideline organized by CFDI as a representative of the industry.
总结,会议结束 Summary , Meeting Adjourned
Speakers

李锦才博士
CEO of 药明合联PPPQMS及其在固体制剂的应用PPPQMS and its application in solid preparations
Speakers

陈四向
专家 at 广东东阳光药业股份有限公司陈女士,制药行业超过三十年的工作经验,历任知名药企质量总监、总经理。曾负责从0到1到超百个产品获批及国内外商业化上市。带领团队通过二十余次的国内GMP检查,以及十余次的美、欧、WHO等国外GMP检查,有丰富的质量管理经验及国际GMP认证经验,也是新版GMP指南《口服固体制剂与非无菌吸入制剂》分册编委;曾与多家国内外知名的设计院合作,组织建设了四家工厂(含制剂及API)。在药厂建设,经营管理方面有丰富的知识和经验积累,是位认真负责,开拓创新的管理者。 Ms. Chen, with over thirty years of work experience in the pharmaceutical industry, she has served as the Quality Director and General Manager of well-known pharmaceutical companies. Promote the approval and commercialization of hundreds of new products. Lead team to pass the domestic GMP inspection for more than 20 times, as well as stringent regulatory authority (e.g. FDA, EMA, and WHO) GMP inspection for more than 10 times. Having rich experience in quality management and international GMP inspection, she is also a member of the editorial board of the new Chinese GMP Guideline "Oral Solid Preparations and Non-Sterile Inhalation Preparations". Collaborated with several well-known design institutes both domestically and internationally to organize the construction of four factories, including drug product and drug substance. With rich knowledge and experience in the construction and management of pharmaceutical factories, she is a conscientious and innovative manager.
国际典型无菌检查缺陷分析及对中国企业的启示 Typical observations analysis in international aseptic inspections and the enlightenment for Chinese enterprises
Speakers

韩亮
北京大学知识工程与监管科学实验室研究顾问Research Consultant, Knowledge Engineering and Regulatory Science Laboratories, BeijingUniversity at 识林知识平台负责人Head of Shlinx韩亮博士,识林知识平台负责人,北京大学知识工程与监管科学实验室研究顾问。从事药品生产质量法规政策和产业质量体系
研究,牵头负责2010版GMP指南撰写和再版修订工作。长期参与IPEM项目的组织和教学。2014年毕业于北京大学。
总结,会议结束 Summary , Meeting Adjourned
Speakers

王卫兵 Alex
副总裁VP at 江苏恩华药业,Jiangsu Nhwa Pharmaceutical Co.,Ltd总结,会议结束 Summary , Meeting Adjourned
Speakers

王卫兵 Alex
副总裁VP at 江苏恩华药业,Jiangsu Nhwa Pharmaceutical Co.,Ltd总结,会议结束 Summary , Meeting Adjourned
Speakers

孙京林
副总裁VP at 中国生物技术股份有限公司, China National Biotec Group Company Limited (CNBG)会议主席致欢迎辞 Welcome Speech by Chair
Speakers

庞飞飞 Sophie
董秘 Board Secretary at 北京鼎持,Beijing Dingchi投行角度分享 Case sharing on Investment Bank Perspecitve
Speakers

吴虹生
执行董事 at 招商证券投资银行委员会中国医药企业赴美投资动因观察 Observation on the motivation of Chinese pharmaceutical enterprises to invest in the United States
Speakers

Rosemary Hu
Partner at 德勤中国 Deloitte China胡晓蕾女士是德勤中国的税务合伙人,拥有超过20年的专业服务经验。胡女士被《国际税收》连续多年收录为杰出税务顾问和杰出女性税务领导,她目前担任江苏省注册税务师协会常务理事以及上海美国商会南京中心副主席。 胡晓蕾女士的专业经验包括为企业集团和大型企业提供整体税务合规和税负优化方案,在企业并购中提供税务架构规划、税务尽职调查、股权和经营结构税务规划。她自2005年开始已为多家境内外公司提供上市税务服务。 胡女士所领导的德勤南京税务团队是“四大”之中在宁历史最久、规模最大的团队,目前是苏皖地区四大中唯一的5A等级税务师事务所。 胡晓蕾女士毕业于南京大学法学院,是中国注册会计师与注册税务师。 Rosemary Hu is the leader of Deloitte Nanjing tax team. She is also a tax partner in International Tax and M&A service line with 22 years of professional tax consulting experiences. She has a diverse background serving MNC, SOE, listed companies, and high potential local large enterprise groups in Jiangsu and Anhui province. She has significant experience across a range of industries. Rosemary was continuously nominated as Highly Regarded Practitioner and Women Leader in Tax by World Tax. She is also an executive director of Jiangsu CCTAA (“The China Tax Certified Tax Agents Association”). Ms. Hu holds a BA from the law School of Nanjing University and she is a member of Chinese Institute of Certified Public Accountants (CICPA) and Certified Tax Agent in PRC. Ms. Hu is the vice chair of Amcham Nanjing Center Advisory Council.
马里兰州助力中国药企出海美国市场 Maryland Offers Support to Chinese Biopharmaceuticals in Entering US Market
Speakers

王艳波Vikki
中国办公室代表China office representative at 美国马里兰州商务厅Maryland Department of Commerce总结 ,会议结束 Summary , Meeting Adjourned
Speakers

庞飞飞 Sophie
董秘 Board Secretary at 北京鼎持,Beijing Dingchi注册方案
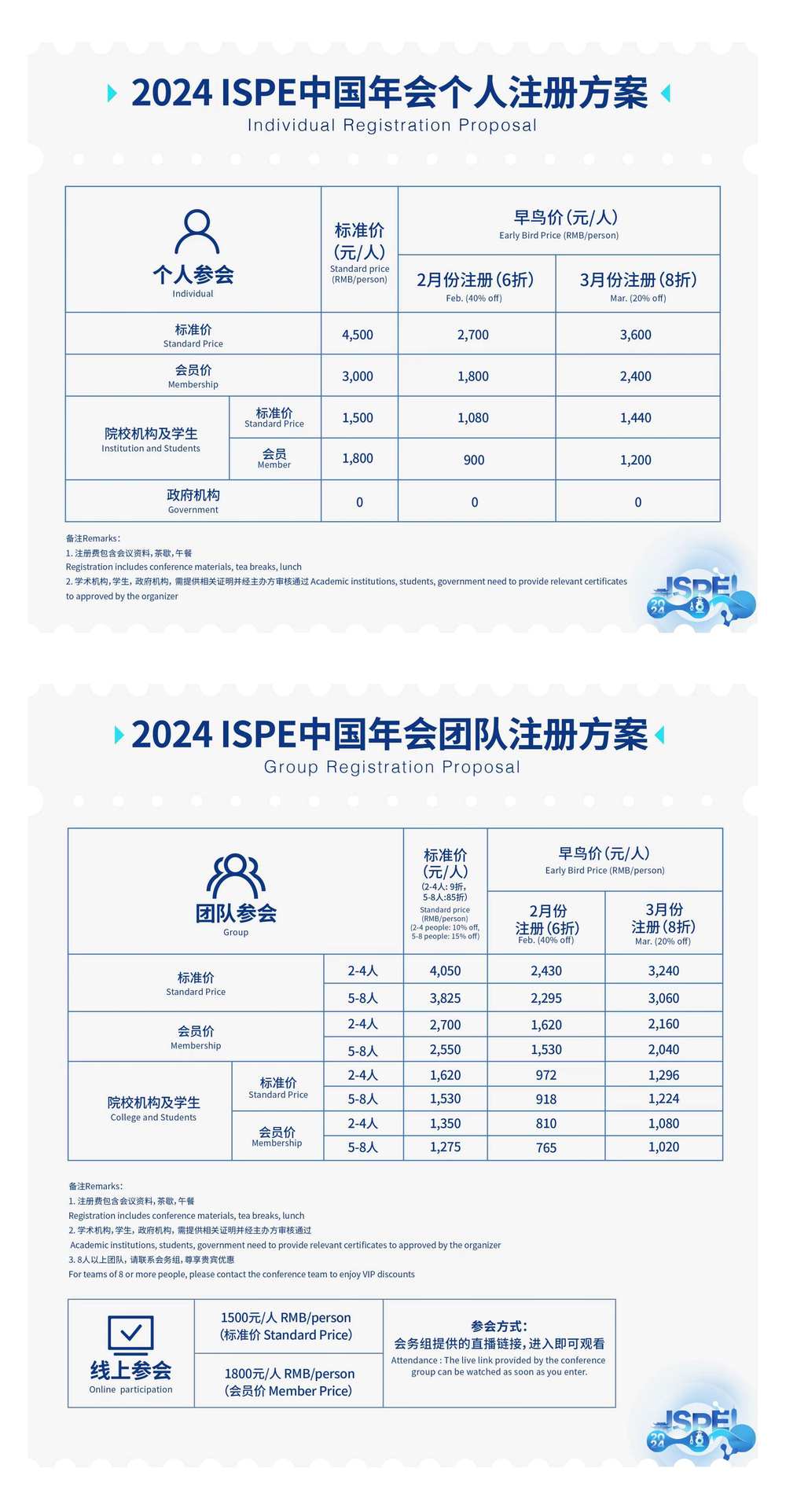
2-4人参会 折扣码:CF90
5-8人参会折扣码:CF85
Speakers

顾晨
资深产品经理Senior Product Management at 星德科包装技术(杭州)有限公司Syntegon Packaging Technology (Hangzhou) Co., Ltd._
More Information

Mr. Maurice B. Parlane , B Tech MIT
Principal/Director of New Wayz Consulting Ltd/CBE Pty Ltd
More Information

Aaron Weinstein
Senior Director, Compliance Consulting of IPS – Integrated Project Services, LLC
More Information

付迪宇Orville
创始人 战略及业务发展负责人Founder and Head of Strategy and Business Development at 华升智药科技开发(北京)有限公司 Huasheng Pharmatech (Beijing) Co., Ltd.
More Information

施维维
中国质量负责人 Head of Quality, at 赛默飞临床试验事业部Thermo Fisher Clinical Trail Division China
More Information

柯桂盛
制剂工艺经理 Production Process Manager at 深圳市海普瑞药业集团股份有限公司Shenzhen Hepalink Pharmaceutical Group Co., Ltd.
More Information

王艳波Vikki
中国办公室代表China office representative at 美国马里兰州商务厅Maryland Department of Commerce
More Information

罗建军J.J. Luo
生物偶联产品研发和生产副总裁VP of Bioconjugate Product Development and Manufacturing at 无锡药明合联生物技术有限公司WuXi XDC Co. Ltd
More Information

茅亚超Robin
联合创始人 产品研发负责人Co-Founder and Head of Product Development at 华升智药科技开发(北京)有限公司Huasheng Pharmatech (Beijing) Co., Ltd.
More Information

韩亮
北京大学知识工程与监管科学实验室研究顾问Research Consultant, Knowledge Engineering and Regulatory Science Laboratories, BeijingUniversity at 识林知识平台负责人Head of Shlinx
More Information

马强
Sales Manager - Analytics at Knick (Shanghai) Electronic Measurement Trading Co., Ltd
More Information

Ping ZHANG
ISPE 中国理事会主席 Chair of ISPE China Committee

Tonia
Bernard
Assistant Country Director助理主任 of FDA China OfficeFDA中国办公室
Tonia Bernard is an Assistant Country Director with the FDA’s OGPS China office, where she performs inspections of pharmaceutical facilities located in China. Prior to joining the FDA China Office, Ms. Bernard has more than 5 years of field experience with FDA’s ORA performing a broad range of domestic and foreign drug inspections. She earned a Master of Public Health in Environmental Science from Fort Valley State University in Fort Valley, Georgia and a Bachelor of Science in Exercise Science from University of Maryland Eastern Shore in Princess Anne, Maryland.
贝妮娜,公共卫生硕士
FDA中国办公室助理主任
贝妮娜在FDA中国办公室任助理主任,开展对中国制药企业的现场检查工作。在加入中国办公室之前,贝妮娜在FDA监管事务办公室(ORA)有超过5年的工作经验,在国内国外进行广泛的药品检查。她在乔治亚州福特谷的福特谷州立大学获得环境科学公共卫生硕士学位,并在马里兰东岸大学获得运动科学学士学位。

Chip Bennett
Associate Director, Global C&Q of CAI
A Project Manager and Senior Validation Engineer, Chip is a PMI® Certified Project Management Professional (PMP), a recognized industry expert, speaker, and published author, and a consultant with over 20 years of experience in the pharmaceutical and regulated non-pharmaceutical industries and with expertise in risk-based verification, aseptic manufacturing, cleaning validation, quality systems, and owner project management. Chip is responsible for developing and implementing Quality Risk Management (QRM) based Commissioning and Qualification programs and projects, with a focus on assessing and training clients regarding development, implementation, and transition to risk-based approaches. Chip was a lead author on the ISPE Good Practice Guide: Good Engineering Practice, 2nd Edition (2021).

李锦才博士
CEO of 药明合联

高杨 博士
首席战略官(CSO) at 上海邦耀生物科技有限公司
高杨博士目前担任上海邦耀生物科技有限公司的首席战略官,全面负责公司基因和细胞治疗产品的开发、申报、项目资产管理等工作,协助CEO整个公司的经营
加入上海邦耀生物科技有限公司之前,高博士在制药行业拥有近20年的管理和开发经验,先后在国家药品监管机构、跨国制药企业、生物科技公司和全球CRO公司担任技术/管理工作,熟悉制药行业全链条的运转之道,尤其在药物开发策略、申报策略、质量体系建设和商业化战略方面有着全面、丰富的经验。曾是中国国家药品监督管理局药品审评中心的高级审评官,8年时间内审评过千份申报项目并执行过几十次现场检查,涵盖跨适应症领域的新药临床申请、新药上市申请、仿制药申请、上市后补充申请等技术审评工作,对监管科学和CDE、FDA、EMA及ICH的技术指南有着全面深刻的理解。之后4年担任礼来公司的法规CMC和医疗器械负责人,领导多个创新药CMC策略、技术转移策略和医疗器械注册策略的制定和执行,参与礼来全球加快研发的改革工作,并为礼来在华合作伙伴的创新药研发和商业化提供技术支持。同期兼任RDPAC的CMC委员会轮值主席,领导7个工作组为2015年启动的中国药监改革积极建言献策,为构建支持药物创新的新生态系统做出了杰出贡献。曾担任苏州亚盛制药的法规事务总监,制定并执行中美研发和注册策略,成功获批中、美多个IND。之后担任PAREXEL的首席药物研发和法规顾问,3年时间内为多达30家国内外生物制药公司约50个项目提供研发和监管策略的咨询服务,成功助力他们在中美获得监管部门的批准并开展新药研发。业余时间,高博士积极探索和实践人工智能和数字技术赋能药物研发,设计并开发远程智能化临床试验平台。

康伟
副总裁VP at 奥星集团 Austar Group
0+ years of ISPE member Member of ISPE China Biological Committee Vice President, Austar, 2019-01 to Present Senior specialist and SME, NNE 2007- 2019
With 28+ years of work experience in biopharmaceutical and pharmaceutical industry as project sponsor and project lead, Mr. Kang Wei takes role as Vice President in AUSTAR Group at present and focus on pharmaceutical engineering and project management. He works on strategy planning and biotech facility engineering as international biotech facility expert with visionary approach. Highlight clients & projects (GMP commercial production facility) as follows: Bill & Melinda Gates Foundation, NovoNordisk, GSK, Novozymes, Boehringer-Ingelheim, Adimmune, Henlius, Wuxi Biologics, Junshi, BeiGene, Carsgen, Gracell.
Mr. Kang Wei coordinated with international team to provide professional consulting services for international and domestic clients in terms of Vaccine, Monoclonal Antibody, ATMP, ADC, etc., the project standards comply with FDA, EMA, WHO and NMPA.
International organizations and forums:
“Establishing Biotech Manufacturing Capacity in China” in 2015, IBC Life Sciences D development and Production (BDP) Week, in Huntington Beach, CA.
“Bio facility consideration from engineering perspective” in 2016, ISPE Biologics Development Symposium in Hangzhou
“Best practice for Bio project execution “in 2017, ISPE Biologics Forum in Benxi
“Global Practice Bridge to Local Manufactures “in 2018, ISPE / CIPM in Chongqing
“THE MANUFACTURING ROAD “Panel discussion in 2019, BioCentury & BayHelix China Summit in Shanghai
“GMP trend and expectation for cell therapy facility” in 2019, ISPE "Innovative technology and concept for state-of-the-art biologic facility" session in Changsha
“Case study - Compliant and Efficient biologic facility “in 2020, ISPE / CIPM in Chongqing

顾晨
资深产品经理Senior Product Management at 星德科包装技术(杭州)有限公司Syntegon Packaging Technology (Hangzhou) Co., Ltd._
工程学硕士学位,12年液体制剂产品相关经验,主要关注隔离器技术产品的开发。
Engineering Master Degree. 12 year pharma liquid machine experience, focus on Isolator technology product development.

娄再飞
验证与合规负责人 at 星德科包装技术(杭州)有限公司
娄再飞 :星德科包装技术(杭州)有限公司 现任验证与合规部门负责人,2011年加入星德科,主要负责制药包装设备的技术文档,验证和法规符合性等工作。曾在国内外知名药企从事药品研发,无菌药品生产验证和法规符合性工作,拥有近20无菌药品生产经验。
Lou Zaifei: Department head of service for validation and compliance in Syntegon Packaging Technology (Hangzhou) Co., Ltd.,. Joined Syntegon in the year of 2011, is mainly responsible for delivering of technical documentation, validation and regulatory compliance of pharmaceutical equipment. Previously worked in famous pharmaceutical company for drug R& D, sterile drug production&validation and compliance. With about 20 years of experience in aseptic process.

Mr. Maurice B. Parlane
, B Tech MIT
Principal/Director of New Wayz Consulting Ltd/CBE Pty Ltd
Maurice Parlane is Principal of New Wayz Consulting Ltd in New Zealand and a Director of CBE Pty Ltd in Australia. He is a professional engineer with 30 years’ experience within the biopharmaceutical industry, including 20 years as an industry consultant providing support to manufacturing and compliance management; validation and operational excellence projects in Australasia and the Asia Pacific region. Prior to this, he held senior engineering and manufacturing roles within the Glaxo group of companies. He has a Bachelor of Manufacturing Technology (Hons) as well as mechanical and electrical engineering qualifications. Maurice is past president and current director of the ISPE Australasian Affiliate. He is the co-lead of ISPE’s Asia Pacific Regulatory and Quality Harmonization committee and leader of the Process Validation Team and is a member of the Guidance Documents Committee. He is an ISPE PV Instructor and was named ISPE Member of the year in 2016. He is currently serving as the Leader of the ISPE Process Validation Team.

Glasser
Barrett
Senior Director, Global Clinical Supply Chain of Takeda
With more than 25 years of experience working in biotech and life sciences, Barrett has long been passionate about the many applications of digital technology to drug development and supply chain domains. After starting his career in technology consulting at Pfizer, he moved into supply chain planning and integration at Biogen for a number of years before joining Takeda and eventually taking on his current role as a Digital Global Clinical Supply Chain leader. During that time, Barrett obtained his MBA from Boston University and his Master of Public Health from Harvard. When not working, Barrett enjoys music, cooking, and spending time with his dog.

Richard CHAI
Senior Technical Service Manager at STERIS
Richard Chai is a Senior Technical Service Manager for the Life Sciences Division of STERIS Corporation. He is an industry speaker at ISPE events (Singapore, Malaysia, Indonesia, Thailand, Philippines, India) and Interphex Japan
Richard先生目前任职STERIS生命科学部门的高级技术服务经理,多次在制药行业会议和展会上作为讲师参与技术交流(如ISPE新加坡、马来西亚、印度尼西亚、泰国、菲律宾、印度和Interphex日本)
Prior to joining STERIS, Richard had 13 years of manufacturing and validation experience working in various pharmaceutical companies. In addition, he has extensive experience in cleaning validation having worked as cleaning validation consultant in various biopharmaceutical and biotech companies
加入STERIS之前,Richard先生有超过13年在制药行业的生产和验证工作的经验,包括曾在多家生物制药公司担任清洗验证咨询师,对许多制药客户在设备验证、产品接触表面的清洗验证等方面提供技术支持

Rosemary Hu
Partner at 德勤中国 Deloitte China
胡晓蕾女士是德勤中国的税务合伙人,拥有超过20年的专业服务经验。胡女士被《国际税收》连续多年收录为杰出税务顾问和杰出女性税务领导,她目前担任江苏省注册税务师协会常务理事以及上海美国商会南京中心副主席。 胡晓蕾女士的专业经验包括为企业集团和大型企业提供整体税务合规和税负优化方案,在企业并购中提供税务架构规划、税务尽职调查、股权和经营结构税务规划。她自2005年开始已为多家境内外公司提供上市税务服务。 胡女士所领导的德勤南京税务团队是“四大”之中在宁历史最久、规模最大的团队,目前是苏皖地区四大中唯一的5A等级税务师事务所。 胡晓蕾女士毕业于南京大学法学院,是中国注册会计师与注册税务师。 Rosemary Hu is the leader of Deloitte Nanjing tax team. She is also a tax partner in International Tax and M&A service line with 22 years of professional tax consulting experiences. She has a diverse background serving MNC, SOE, listed companies, and high potential local large enterprise groups in Jiangsu and Anhui province. She has significant experience across a range of industries. Rosemary was continuously nominated as Highly Regarded Practitioner and Women Leader in Tax by World Tax. She is also an executive director of Jiangsu CCTAA (“The China Tax Certified Tax Agents Association”). Ms. Hu holds a BA from the law School of Nanjing University and she is a member of Chinese Institute of Certified Public Accountants (CICPA) and Certified Tax Agent in PRC. Ms. Hu is the vice chair of Amcham Nanjing Center Advisory Council.

Goebel
Martin
Sales Director Analytics of Knick GmbH, Germany
Personal Introduction: With a master degree in chemical engineering from University of Karlsuhe/Germany, Martin started as sales and project engineer for process water and ultra pure plants on international projects. After 5 years working in South East Asia on process water projects he moved to the analytical field focussing on TOC analyzers for ultra pure pharma water and process water for various industries. Martin was involved in many validation processes for TOC analyzer integration into pharma water plants in Europe. Since 5 years Martin is sales director with Knick, Berlin, Germany and responsible for integration of pH analyzers into industrial processes. His special field are applications in bio-pharma industry.

Dr. Rainer
Nicolai
Product Owner Engineering Consulting of F. Hoffmann - La Roche AG
Dr. Rainer Nicolai is the product owner Engineering Consulting at Roche’s headquarter site at Basel/Switzerland. He has worked as a project manager for more than 20 years and is a subject matter expert on containment technologies covering the whole manufacturing value chain.

Ms. Catherine T.
Oakes
Managing Director of Oakes Group Global Ltd.
Catherine Oakes is an internationally recognized quality and compliance professional with 30+ years of diverse experience in the regulated healthcare and life sciences industries. She has held many senior and executive management positions in clinical laboratory, pharmaceutical, biotech, diagnostic, and medical device establishments in the U.S. and around the globe. Catherine is the Managing Director and Principal Consultant for Oakes Group Global where she works on the front line with industry, professional associations, health agencies, and regulatory authorities worldwide. Catherine holds a Bachelor of Health Services Administration from Florida Atlantic University and a Master of Science in Jurisprudence from Seton Hall University School of Law with a dual concentration in Pharmaceutical & Medical Device Law & Compliance and Health & Hospital Law.

Jeff Odum
Practice Leader: ATMPs & Biologics at Genesis AEC
Jeff Odum’s career encompasses over 30 years of experience in the development, execution, delivery, and management of facility and operational programs in the biotechnology and pharmaceutical industries. During his career, he has been involved in leading and managing strategic planning, feasibility and conceptual planning/design, design, construction, start-up, and validation support, in addition to participating in the development and presentation of licensing packages to the FDA. Specific responsibilities have included positions as Global Lead, Operations Director, Design Manager, Facility/Engineering Manager, Owner’s Representative, Program Manager, and Construction Site Manager for projects focused on the manufacture of human therapeutics including vaccines, cancer therapeutics, generics, and ATMPs. These efforts ranged in size from $1 million to more than $2 billion in total program costs.
Jeff was the North American Education Advisor to ISPE, where he also is currently the Chair of the Global Biotechnology Community of Practice and the Project Lead for the ATMP Guide for Autologous Cell Therapy Products, co-Lead for the new revision of the Biotech Manufacturing Facilities Baseline Guide, chapter author for the Biotech Product and Process Development Baseline Guide, and a lead author for the BPOG Closed Systems Closure Playbook. He has written over 50 Technical articles and published four Industry reference books.
He is a member of the BTEC teaching faculty in NC State University’s Gradute program in Biomanufacturing.

Smith Paul
Global Compliance Specialist at Agilent Technologies
Paul has worked in a range of quality, laboratory, management and consulting roles over his +37 year career. He started as an analytical specialist, interpreting FT-IR and FT-Raman spectra, participating in research studies using these techniques, and chemometric modelling of physical properties against the spectra (as part of his MSc research studies). He then moved into broader Analytical Chemistry, Technology Transfer, and Project Management work. He was a Laboratory Operations Manager in GSK in 2002 when he left to become an independent consultant. As part of his consultancy work, he Lectured in Quality and Technology Transfer in the Pharmaceutical Industry at Greenwich University. During his career, he completed his first software validation in 1994 and has contributed to GAMP Good Practice guides and 20+ articles related to laboratory compliance and is a member of the ISPE GAMP UK Community of Practice (CoP) Steering committee. He has presented at ISPE events in China, Singapore, and the UK and other conferences and events all over the world. Paul has worked for Agilent for over 12 years and much of his recent work has focused on laboratory compliance - monitoring and tracking non-compliance information (e.g., FDA findings), regulatory trends, changes, and evolution in this area. He is currently formally reviewing GAMP Good Practice Guides (to determine if updates are required). His passion for transforming and visualizing regulatory data (to turn into data insights) comes from his early career and chemometric modelling. As part of his current Agilent role, Paul supports Agilent’s global compliance council of laboratory specialist and creates insightful content and presentations to help educate and inform laboratory managers and compliance leaders. He holds a BSc in Chemistry and an MSc in Analytical Chemistry (more information can be found in Paul’s Linked In profile – which is kept up to date).

Zhihao Peter
Qiu
External Advocacy Lead APAC at Roche Genentech.
裘博士曾任美国FDA CDER药品生产评估办公室生物技术制造部门负责人。在CDER,他负责监督生物制品许可申请的生产控制和设施的科学审查和质量评估。他的部门还负责对CDER监管的生物制品进行许可前/批准前检查。裘博士是全球领先的生物技术生产微生物控制和无菌保证评估专家。在加入CDER之前,他还曾在美国FDA生物制品评估与研究中心(CBER)担任CMC设施审查员/检查员,并在器械与放射卫生中心(CDRH)担任毒理学副主任。
裘博士毕业于南加州大学,获得生物科学博士学位,在加入Roche Genetech之前,曾在信达生物制药(苏州)有限公司担任首席质量官。
Dr. Qiu was a Division Director in the Division of Biotechnology Manufacturing, Office of Pharmaceutical Manufacturing Assessment, CDER, USFDA. At CDER, he oversaw the scientific review and quality evaluation of the manufacturing controls and facilities for Biologics license applications. His Division was also responsible for conducting pre-license/pre-approval inspections for CDER regulated biological products. Dr. Qiu is a global leading expert in microbial controls and sterility assurance assessment for biotech manufacturing, and also has extensive knowledge of FDA regulatory requirements in manufacturing facility assessments and GMP compliance activities. Prior to CDER, he also worked in FDA’s Center for Biologics Evaluation and Research (CBER) as a CMC facility reviewer/inspector and in the Center for Devices and Radiological Health (CDRH) as an associate director for Toxicology.
Dr. Qiu graduated from the University of Southern California with a Ph.D. in Biological Sciences and worked at Innovent Biologics (SuZhou) as the Chief Quality Officer before joining Roche Genentech.

Mark
Stephens
SME at Exyte (Shanghai) Co., Ltd.
Mark,Exyte生物制药&生命科学课题专家,具有丰富的生物制药设施项目经验,主导过澳洲、欧洲、中国等多地国际知名项目,为诺华、默克、辉瑞等企业提供服务,致力于为生命科学领域的发展贡献力量。
Mark is a seasoned professional in the Biopharma & Life Sciences sector, with extensive expertise in designing and constructing biopharmaceutical facilities. He has a proven track record of effectively overseeing prominent projects in Australia, Europe, and China, and has established collaborative partnerships with industry leaders such as Novartis, Merck, Pfizer, and other key players. Mark's unwavering commitment to excellence, combined with his profound knowledge, drives his mission to lead the industry forward and foster continuous progress.

John Vaughn
Vice President, Asia of CAI
John serves as the Vice President, Asia Life Sciences for CAI. With progressive responsibility within the organization over the past ten years, he has served as project manager, client manager, area client manager, sales manager, and regional director, including four years in Asia Pacific. Over his 20-year career, John’s technical background is in process calibration and metrology, validation, and computerized asset management systems. With previous roles as calibration manager and C&Q manager and extensive field experience in CQV along with ISA certifications in instrumentation and controls, he brings a strong level of technical competence to leadership roles.

Aaron
Weinstein
Senior Director, Compliance Consulting of IPS – Integrated Project Services, LLC
Ms. Stephanie A.
Wilkins, PE
President of PharmaConsult US Inc
Stephanie Wilkins, PE, Lean Six Sigma Green Belt, has over 30 years of professional experience in project management, risk management, engineering and validation solutions for the pharmaceutical and biotech industry including research, development, and pilot plant and manufacturing facilities. She is president of PharmaConsult US, Inc, which provides cross-contamination and containment consulting to the pharmaceutical industry. Wilkins, a member of ISPE since 1993 is the co-Chair of the ISPE Risk-MaPP Baseline Guide Task Team and was a member of the ISPE International Board of Directors. Wilkins is an ISPE trainer for Managing Cross Contamination and Risk-MaPP Principles. Wilkins also is a reviewer for articles to be placed in the Pharmaceutical Engineering Journal as well as contributed articles, given lectures and organized courses for ISPE. Wilkins graduated from the Pennsylvania State University with a Bachelor of Architectural Engineering.

丛征宇
高级副总裁 at 南京驯鹿医疗技术有限公司
现任南京驯鹿生物技术有限公司高级副总裁,主要职责包括供应链管理、仓储物流、采购、人力资源、信息技术。
在此之前,曾任默沙东中国供应链负责人、杜邦亚太区精益生产体系导师、诺华化学运营供应链负责人、勃林格殷格翰大中华供应链负责人、复星凯特供应链负责人。
拥有16年医药供应链管理和11年运营管理经验,在化药和细胞治疗供应链管理、世界级供应链体系、精益生产体系方面拥有深厚的理论和实践经验。曾两度带领团队完成Oliver Wight世界级供应链项目:默沙东卓越运营项目;诺华NOSSCE项目。

付迪宇Orville
创始人 战略及业务发展负责人Founder and Head of Strategy and Business Development at 华升智药科技开发(北京)有限公司 Huasheng Pharmatech (Beijing) Co., Ltd.
付迪宇 拥有超过13年的跨国医药和生物科技企业创新药临床试验供应链管理经验。他曾带领团队负责多个全球及区域临床试验供应链的策略设计,并成功实施,支持新药在欧洲、美国、亚太等多个重要国家和地区上市,展现了他卓越的领导能力和深厚的专业知识。作为曾在百济神州生物科技的临床供应管理副总监和辉瑞临床试验供应的高级经理,付先生在行业内建立了显著的成就。付先生于2021年北京大学光华管理学院攻读MBA,师从管理科学与信息系统系彭一杰教授,这一经历不仅为他提供了坚实的供应链仿真优化理论基础,也激发了他在该领域的创新思维和战略规划能力。
近期,他和几位跨行业领导者联合创立华升智药科技开发(北京)有限公司 目前担任战略及业务发展负责人。华升智药是一家深度科技公司,通过使用基于蒙特卡洛模拟、贝叶斯算法等技术开发的仿真模拟平台及专业技术服务,为各类制药公司、生物科技公司、研发合同性组织(CRO)提供专业的供应链咨询服务及全链条优化的解决方案。
Orville Fu has over 13 years of experience in clinical trial supply chain management for innovative drugs within multinational pharmaceutical and biotech companies. He has led teams in strategy development and successful implementation of global and regional clinical trial supply chains, until the approval of new drugs in key countries and regions including Europe, the United States, and Asia-Pacific, demonstrating his outstanding leadership abilities and deep professional knowledge. As the former Associate Director of Global Supply Chain at BeiGene and Senior Manager of Clinical Trial Supply at Pfizer, Mr. Fu has established significant achievements in the industry. Mr. Fu pursued an MBA at Guanghua School of Management, Peking University in 2021, under Professor Peng Yijie of the Department of Management Science and Information Systems. This experience not only provided him with a solid foundation in supply chain simulation optimization theory but also inspired innovative thinking and strategic planning capabilities in the field.
Recently, with several leaders from the industry, he co-founded Huasheng Pharmatech (Beijing) Co., Ltd., where he currently serves as the Head of Strategy and Business Development. Huasheng Pharmatech is a deep-tech company that offers professional supply chain consulting services and comprehensive optimization solutions to pharmaceutical companies, biotech companies, and Contract Research Organizations (CROs) through a simulation platform and professional technical services developed vial technologies like Monte Carlo simulation and Bayesian algorithms.

何永江
Senior Project Manager, Enterprise Solutions企业解决方案高级项目经理 at Cytiva
何永江毕业于天津大学,有着14年以上的制药工程行业工作经验, 并取得过PMP/6σ黑带及CWI等国际认
证。曾就职于颇尔过滤器有限公司先后担任过工艺工程师及项目经理。现作为Cytiva企业解决方案高级项
目经理任职于Cytiva企业解决方案部门,拥有着丰富的相关工艺知识和行业项目管理经验并成功管理参与
过多个客户企业解决方案项目。
Yongjiang He gratitude from Tianjin University, have more than 14 years of experience and is
PMP/ Six Sigma BB/ CWI certified. He has worked as a process engineer and project manager in
Pall Now as a senior project manager of Cytiva Enterprise Solutions, he is working in Cytiva
Enterprise Solutions department, with rich process knowledge and industry project
management experience and has successfully managed and participated in many customer
enterprise solution projects.

刘雅容,Ph.D
CEO of 沙砾生物
刘雅容, 美国南加州大学博士、博士后,沙砾生物创始人、首席执行官,中山大学孙逸仙纪念医院逸仙医学客座教授;江苏省双创人才、姑苏领军人才、苏州工业园区科技领军人才、上海市海外高层次人才专家、上海市浦江人才;正高级研究员,CSCO会员,女医师协会会员,浦东新区青年联合会委员;拥有10余年从事腺病毒、慢病毒等各种病毒载体用于定向基因治疗的研究经验,以及慢病毒产业化的经验;在Nature Communications, Science Immunology,Gene Therapy,Molecular Therapy 等国际顶级杂志发表40多篇论文。并拥有1项美国技术专利,申请细胞治疗领域发明专利90余项,主持上海市生物医药科技支撑项目1项,作为第二完成人获得2017年浦东新区科技进步奖创业团队三等奖;2022年浦东新区青年优秀企业家、2021年获得上海市创业新秀、长三角女性创新创业大赛银奖。2019年创立沙砾生物,致力于创新细胞疗法TIL在实体瘤的研发和临床应用。
Yarong Liu, Ph.D., postdoctoral fellow, University of Southern California, founder and CEO of Grit biotechnology, Visiting Professor of Yixian Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University; Double Innovation talents of Jiangsu Province, leading talents of Gusu, leading talents of Science and Technology of Suzhou Industrial Park, experts of overseas high-level talents of Shanghai, talents of Shanghai Pujiang; Senior researcher, Member of CSCO, member of Women's Medical Association, member of Pudong New Area Youth Federation; She has more than 10 years of experience in the research of various viral vectors such as adenovirus and lentivirus for targeted gene therapy, and experience in the industrialization of lentivirus. She has published more than 40 papers in top international journals such as Nature Communications, Science Immunology, Gene Therapy, Molecular Therapy, etc. She has one American technology patent, applied for more than 90 invention patents in the field of cell therapy, presided over one project of Shanghai biomedical science and technology support, and won the third prize of the entrepreneurship team of Pudong New Area Science and Technology Progress Award in 2017 as the second complete person; In 2022, Pudong New Area Young Outstanding Entrepreneurs, and in 2021, won the Silver Award of Shanghai Start-up Rookie and Yangtze River Delta Women Innovation and Entrepreneurship Competition. In 2019, She found Grit biotechnology, which is committed to the development and clinical application of innovative cell therapy TIL in solid tumors.

刘雅容,Ph.D
CEO of 沙砾生物
刘雅容, 美国南加州大学博士、博士后,沙砾生物创始人、首席执行官,中山大学孙逸仙纪念医院逸仙医学客座教授;江苏省双创人才、姑苏领军人才、苏州工业园区科技领军人才、上海市海外高层次人才专家、上海市浦江人才;正高级研究员,CSCO会员,女医师协会会员,浦东新区青年联合会委员;拥有10余年从事腺病毒、慢病毒等各种病毒载体用于定向基因治疗的研究经验,以及慢病毒产业化的经验;在Nature Communications, Science Immunology,Gene Therapy,Molecular Therapy 等国际顶级杂志发表40多篇论文。并拥有1项美国技术专利,申请细胞治疗领域发明专利90余项,主持上海市生物医药科技支撑项目1项,作为第二完成人获得2017年浦东新区科技进步奖创业团队三等奖;2022年浦东新区青年优秀企业家、2021年获得上海市创业新秀、长三角女性创新创业大赛银奖。2019年创立沙砾生物,致力于创新细胞疗法TIL在实体瘤的研发和临床应用。
Yarong Liu, Ph.D., postdoctoral fellow, University of Southern California, founder and CEO of Grit biotechnology, Visiting Professor of Yixian Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University; Double Innovation talents of Jiangsu Province, leading talents of Gusu, leading talents of Science and Technology of Suzhou Industrial Park, experts of overseas high-level talents of Shanghai, talents of Shanghai Pujiang; Senior researcher, Member of CSCO, member of Women's Medical Association, member of Pudong New Area Youth Federation; She has more than 10 years of experience in the research of various viral vectors such as adenovirus and lentivirus for targeted gene therapy, and experience in the industrialization of lentivirus. She has published more than 40 papers in top international journals such as Nature Communications, Science Immunology, Gene Therapy, Molecular Therapy, etc. She has one American technology patent, applied for more than 90 invention patents in the field of cell therapy, presided over one project of Shanghai biomedical science and technology support, and won the third prize of the entrepreneurship team of Pudong New Area Science and Technology Progress Award in 2017 as the second complete person; In 2022, Pudong New Area Young Outstanding Entrepreneurs, and in 2021, won the Silver Award of Shanghai Start-up Rookie and Yangtze River Delta Women Innovation and Entrepreneurship Competition. In 2019, She found Grit biotechnology, which is committed to the development and clinical application of innovative cell therapy TIL in solid tumors.

吴虹生
执行董事 at 招商证券投资银行委员会

周臻弘
质量副总裁 at 浙江海正药业
Vice President of Quality at Zhejiang Hisun Pharmaceutical Co., Ltd., has worked in multiple departments including production, R&D, sales, and quality, especially in quality department for over 20 years. He has served as the editorial board member for the Quality Management System sub-volume and API sub-volume of the Chinese GMP Guidelines (2nd edition). 目前担任浙江海正药业质量副总裁,历经生产,研发,销售,质量等多个部门,在质量部门工作超过20年,担任中国药品 GMP指南(第2版)质量管理体系分册和原料药分册的编委工作。

夏禄华
副总经理 at 江苏金迪克生物科技有限公司
夏禄华,现任职于江苏金迪克生物科技有限公司副总经理,主要从事质量管理工作,先后在海正药业、贝朗医疗、药明生物,康希诺生物工作,负责药品生产质量管理工作。作为工业界代表参与了国家药监局组织的《药品共线生产质量管理指南》,《临床试验用药品生产质量管理规范》,《ICHQ9质量风险管理(R1)》,《抗体类药物现场检查指南》,药品GMP指南-无菌制剂分册等指南的起草和修订。国家药监局高级研究学院特聘专家,浙江省药品审评核验中心特聘专家,上海市药监局特聘专家。

孙京林
副总裁VP at 中国生物技术股份有限公司, China National Biotec Group Company Limited (CNBG)

孙英强
质量经理 Quality Manager at 步长药业/Buchang Pharmaceutical
Personal introduction:Mr. Sun Yingqiang, the quality manager of Buchang Pharmaceutical, has been engaged in the production quality management of traditional Chinese medicines (oral preparations and traditional Chinese medicine injections) for a long time. He is an expert in building systems and promoting the improvement of quality management. He has hosted numerous regulatory on-site inspections for sterile products, and has accumulated rich practical experience. Specially appointed lecturer of the Medical Association, and participated in the editorial board of the university textbook "Drug Analysis". As a representative of the enterprise, I have participated multiple times in the drafting and revision of national regulations, such as the Special Provisions on the Supervision of Traditional Chinese Medicine Production.
孙英强先生,步长制药质量经理,长期从事中成药(口服制剂与中药注射剂)生产质量管理,擅长中成药质量体系搭建和推动质量管理水提升,多次接受无菌产品的现场检查,积累了丰富的实战经验。医药协会特聘讲师,作为编者出版高校教材《药物分析》,作为企业代表多次参与国家法规指南,如《中药生产监督专门规定》等的编写修订工作。

庞飞飞 Sophie
董秘 Board Secretary at 北京鼎持,Beijing Dingchi

张新
顾问教授Consultant professor at 沈阳药科大学Shenyang Pharmaceutical University
张新女士,沈阳药科大学顾问教授,制药工程正高级工程师,质量工程师,六西格玛黑带。曾在国内多家知名制药企业担任质量负责人,质量受权人和工厂运营负责人;擅长质量体系搭建和推动质量管理水平提升,多次接受国内及FDA的无菌产品现场检查,积累了丰富的实战经验。多次赴美欧参加ISPE、PDA等组织的质量管理培训和学术研讨会议;多次应邀赴东南亚、中东国家讲解ICHQ10及GMP相关指南;作为第一副主编,出版高校教材《药品生产质量管理规范》(案例版);作为工业界代表参与了国家药监局审核查验中心组织的《ICH Q9质量风险管理(R1)》工作组工作及新版GMP指南的修订工作。Ms. Zhang Xin is a consultant professor at Shenyang Pharmaceutical University. She is a professorate senior engineer in pharmaceutical engineering, a quality engineer, and a six sigma black belt. She has served as the head of quality, the qualified person and the head of operation in many famous pharmaceutical companies in China. She is an expert in building quality systems and promoting the improvement of quality management. She has hosted numerous regulatory on-site inspections for sterile products by both NMPA and FDA, and has accumulated rich practical experience. She has been to the United States and Europe for multiple times to participate in quality management training and academic seminars organized by ISPE, PDA, etc. She has been invited to the Southeast Asia and the Middle East countries for multiple times to give lectures on ICHQ10 and GMP related guidelines. As the first deputy editor-in-chief, she has published the textbook for colleges and universities, Good Manufacturing Practice (Case Studies). she has taken part in the work of the working group of ICH Q9 Quality Risk Management (R1) and the revision of the new version of the GMP Guideline organized by CFDI as a representative of the industry.

张龙浩
生物工艺部技术支持经理 at 赛默飞世尔科技
赛默飞生物工艺部一次性生物工艺技术经理,在工艺过程控制领域拥有超过13年的行业经验,2018年加入赛默飞生物工艺担任产品技术专家,专注于一次性技术和自动化解决方案,负责一次性生物反应器、一次性工艺容器以及一次性层析系统和一次性离心机等工艺装备在生物工艺上下游的应用和推广,参与过多家头部CDMO以及生物药企一次性生物反应器GMP生产线的新建和运行。

敏凤 Minfeng XU
徐
产品总监Chief Product Officer at 麦克微尔(上海)科技有限公司 Micronview
麦克微尔(上海)科技有限公司环境监测全系列产品总监,包括监测仪器和数字化、智能化解决方案等。徐敏凤近30多年均从事无菌医药生产管理和医药行业合规管理工作,是洁净室(区)和相关设施验证专家,环境监控及风险控制专家。洁净检测国家标准GB/T 16292-16294《医药工业洁净室(区)悬浮粒子、浮游菌和沉降菌的测试方法》项目负责人和主要起草人,翻译出版了有关专著《Thermal Validation in Moist Heat Sterilization》(湿热灭菌工艺的验证)和《Aseptic and Sterile Processing Control, Compliance and Future Trends》(无菌与灭菌-工艺控制与合规及未来趋势)等。

徐禾丰
行业专业人士 Industry professional
徐禾丰,从1984年开始从事制药行业工作,先后从事过生产车间工作、国际贸易工作、注册与质量管理工 作,咨询工作,现在已经退休。先后编写多个FDA、EDMF、COS/CEP等注册文件;翻译了大量的FDA、EU、ICH、WHO、PIC/S、PDA等机构和组织相关法规、指南;翻译出版了许多GMP法规相关的书籍,发表了大量的GMP法规相关论文。先后为各地药监局GMP检查员做了多次培训。
Xu Hefeng began to work in the pharmaceutical industry in 1984, and has been engaged in production workshop work, international trade work, registration and quality management work, consulting work, and is now retired. Has written several FDA, EDMF, COS/CEP and other registration documents; Translated a large number of FDA, EU, ICH, WHO, PIC/S, PDA and other agencies and organizations related regulations and guidelines; He has translated and published many books related to GMP regulations, and published a large number of papers related to GMP regulations. Has done many training for the GMP inspectors of the local drug administration.

施维维
中国质量负责人 Head of Quality, at 赛默飞临床试验事业部Thermo Fisher Clinical Trail Division China
Vela于2014年加入赛默飞Patheon制药服务事业部临床试验服务苏州工厂,目前担任临床试验服务中国区质量负责人,主要负责维护并改进赛默飞临床试验服务中国区的质量管理体系,从而确保临床药品的持续供应和妥善管控,使其符合客户的预期用途、满足客户的规格要求和质量标准。Vela具有药学专业背景,同时因有丰富的质量管理合规经验,拥有15年以上医药质量管理相关工作经验 。

朱人
Golder IH业务负责人 Associate Director, Environment of 科进公司WSP
William Zhu is a Certified Industrial Hygienist (CIH) and Associate Director of WSP Environment China. He has extensive experience in potent compound industrial hygiene/ occupational health risk management including risk assessment, containment design, containment performance assessment. He provides consulting services to multinational and local companies in the pharmaceutical industry. He has a bachelor's degree in Preventive Medicine from Fudan University in 2002 and a master's degree in occupational health in 2005. 朱人是一名美国注册工业卫生师(CIH),科进柏诚环境服务上海办公室副技术总监。他在活性药物成分的工业卫生/职业健康风险管理方面具有丰富的经验,包括风险评估、密闭设计、密闭性能验证等。他为制药行业的国际和国内公司提供咨询服务。他2002年获得复旦大学预防医学学士,2005年获得劳动卫生学硕士。

林森
上游工艺解决方案技术总监 at 默克

柯桂盛
制剂工艺经理 Production Process Manager at 深圳市海普瑞药业集团股份有限公司Shenzhen Hepalink Pharmaceutical Group Co., Ltd.
柯桂盛: 2018年加入深圳市海普瑞药业集团股份有限公司 现任制剂工艺经理,主要负责工艺转移、工艺优化、变更及验证等工作。参与多条液体制剂线建设,项目通过中国、欧盟、美国的GMP认证;参与了“药品GMP指南(第2版)无菌制剂分册”编写工作。 Ke Guisheng: Joined Shenzhen Hepalink Pharmaceutical Group Co., Ltd. in 2018. Currently serving as the Manager of Production Process, mainly responsible for process transfer, process optimization, changes, and validation. Participated in the construction of multiple liquid formulation lines. Projects have passed GMP certification in China, the European Union, and the United States. Also participated in the compilation of the "Drug GMP Guidelines (2nd Edition) Aseptic Formulation Volume."

温源
全球生产部MFG1和MFG4的负责人 at 药明生物
Dr. Yuan Wen is the head of MFG1 and MFG4 of Global Manufacturing of WuXi Biologics. His team was the first in Asia to implement the 4000L single-use bioreactor in GMP and commercial manufacturing approved by ANVISA and EMA. In addition, his team led China’s first 2000L-scale GMP production for mAb using the concentrated fed-batch (CFB) process. Prior to his role in MFG, he was responsible for Cell Culture Process Development at the Wuxi site, with a focus on late phase development and process characterization. Dr. Wen is also an experienced CMC leader to support activities from IND to commercial manufacturing.
Before joining WuXi Biologics, he worked at Life Technologies and Thermo Fisher Scientific in the US, with increasing technical and business responsibilities.
He holds a B.S. degree in Bioengineering and from Chu Kochen Honors College at Zhejiang University, and a Ph.D. degree in Chemical and Biomolecular Engineering from The Ohio State University.
温源博士是药明生物全球生产部MFG1和MFG4的负责人。他的团队是亚洲第一个在GMP商业化生产中使用4000L一次性生物反应器的团队,并获得ANVISA和EMA的批准。他的团队还领导了中国首个2000L规模的单抗GMP生产,采用了浓缩补料(CFB)工艺。在加入MFG之前,他负责无锡基地的细胞培养工艺开发,重点是后期开发和工艺表征。温博士也是一位经验丰富的CMC负责人,支持从IND到商业化生产的活动。
在加入药明之前,他曾在美国的Life Technologies和Thermo Fisher Scientific工作,承担了越来越多的技术和商务责任。
他毕业于浙江大学生物工程和竺可桢荣誉学院,并有俄亥俄州立大学化学和生物分子工程博士学位。

潘海龙
质量管理部主任 at 中国生物技术股份有限公司
生物制品质量管理、生产管理、质量控制
Quality management production management and quality control for biological products
2002.09~2008.09,成都生物制品研究所质量保证部经理助理
Quality Assurance Department Assistant of Chengdu Institute of Biological Products Co.,Ltd.
2008.09~2017.09,成都生物制品研究所质量检定室主任
Quality Control Department Manager of Chengdu Institute
2017.09~2019.08,成都生物制品研究所细菌性疫苗室主任
Bacterial Vaccine Department Manager of Chengdu Institute
2019.08~2020.05,成都生物制品研究所生产管理部经理
Production Management Department Manager of Chengdu Institute
2020.5~至今 中国生物技术股份有限公司质量管理部主任
2020.5 till now, Quality Management Department Director of China National Biotec Group Co., Ltd..

牛萍
齐鲁制药 at 质量与合规高级顾问
制药工程硕士,执业药师,高级工程师(教授级)。从事制药行业质量管理和质量控制三十多年,曾在国内多家著名制药企业(外企及国企)担任质管部部长、质量总监等职。多次接受国内GMP检查、欧盟及美国FDA检查,并获得优异成绩。曾赴荷兰和法国制药工厂,协助中国药监局完成海外GMP检查。参与了药监局组织的“药品生产验证指南”等文件编写工作。 Master of Pharmaceutical Engineering. Licensed Pharmacist, Senior Engineer (professional level). Worked in pharmaceutical quality management and quality control in the pharmaceutical industry for more than 30 years. Served as Quality Manager and Quality & Compliance Director at some famous Pharmaceutical Company. Received domestic GMP inspection, the EU and FDA CGMP inspection many times, and obtained excellent results. Has been to pharmaceutical plants in the Netherlands and France to assist the China Drug Administration in completing overseas GMP inspections. Involved to write GMP related guidelines such as 《 Pharmaceutical Manufacturing Validation Guideline》etc., which organized by China NMPA.

王卫兵 Alex
副总裁VP at 江苏恩华药业,Jiangsu Nhwa Pharmaceutical Co.,Ltd

王晓明
首席顾问 Principle Consultant at 青岛瓦格纳生物咨询有限公司 QD Wagner Biotech Consultant Inc.
QD瓦格纳生物 首席顾问 在纽约瓦格纳学院获得微生物学并于1992年硕士学位,先后在美国SGS、强生和礼来公司从事微生物质量控制。2015年,被礼来公司总部派到礼来苏州制药有限公司负责无菌保障。2018年6月至2022年6月,任信达生物制药公司QC微生物执行总监及MST无菌保障专家。曾任美国PDA(注射药协会)大都会分部的司库,工业微生物协会新泽西分会副会长,纽约瓦格纳学院微生物系兼职教授。著有《制药工业微生物控制及无菌保障》《细菌内毒素》。 QD Wagner Biotechnology Consultant Inc., Principle Consultant Studied in Wagner College in New York and received master's degree in Microbiology in 1992. Worked in Quality Control microbiology lab at SGS, Johnson & Johnson and Eli Lilly in the United States. In 2015, assigned to Eli Lilly Suzhou Pharmaceutical Co., Ltd. by Lilly Headquarters to be responsible for sterility assurance. From June 2018 to June 2022, served as Executive Director of QC Microbiology and MST aseptic Assurance Expert of Innovent Biologics Company. Served as former Treasure of the PDA Metro Chapter, Vice President of the New Jersey Chapter of the Industrial Microbiology Society, and an adjunct professor in the Department of Microbiology at Wagner College in New York. She is the author of two books - "Microbial Control and sterility Assurance in Pharmaceutical Industry" and "Bacterial Endotoxin".

王永增 James
首席技术官 (CTO) at 合源生物
诺华全球细胞治疗质量审核师
美国斯隆·凯特琳纪念癌症中心(MSKCC) 细胞疗法生产运营及质量负责人
美国Vivaldi Biosciences公司GMP产品经理
耶路撒冷希伯来大学分子病毒学博士
美国康奈尔大学及爱荷华州立大学分子病理学博士后
国际细胞治疗协会会员(ISCT)
美国单采协会会员(ASFA)
拥有拥有十多年CAR-T细胞治疗行业经验,包括临床研发、工艺开发、GMP生产、产品商业化、合作医院对接,和全流程质量管理等。

王艳波Vikki
中国办公室代表China office representative at 美国马里兰州商务厅Maryland Department of Commerce

王麟Lynn
Head of Project and Alliance at 药明合联 WuXi XDC Co. Ltd

甘益民
集团副总经理/子公司总经理 at 上海复旦张江生物医药股份有限公司
30年制药企业生产运营管理经验。先后在外资制药企业、民营制药企业及上市制药企业担任生产经理、集团副总经理及子公司总经理。
多次到欧、美、日等国家学习制药行业生产运营管理。对制药行业现代管理有深刻理解和丰富的实践经验。荣获上海市实施发明成果优秀企业家,区拔尖人才。

罗建军J.J. Luo
生物偶联产品研发和生产副总裁VP of Bioconjugate Product Development and Manufacturing at 无锡药明合联生物技术有限公司WuXi XDC Co. Ltd

肖志坚
质量负责人 at 百济神州中国
南京大学生物化学系毕业,中欧全球EMBA,现任百济神州中国质量负责人,历任诺华亚太、中东、非洲和日本区域国家质量部负责人,阿斯利康亚太地区质量负责人等。熟悉全球质量体系,有丰富的跨国团队领导的经验

胡仲新 Jessica
资深质量总监Sr. Director, Quality of 百特上海医疗仪器有限公司Baxter Healthcare
胡仲新女士 1995 年毕业于中国药科大学,先后在武田制药,中美史克,北京诺华制药,罗氏诊
断和百特中国投资从事质量控制和质量管理工作,熟悉欧盟 GMP/GDP,世界卫生组织
GMP/GDP,澳大利亚 GMP,ICH/PICS 指南,韩国 GMP,台湾 GMP/GDP 及中国 GxP 等药品相
关法规要求,ISO 13485,中国医疗器械 GMP 及相关附录, 21CFR part 820, MDR, IVDR 等医疗
器械相关法规要求。在过往 23 年药品行业和 5 年医疗器械行业质量管理过程中,多次带领团队
顺利通过欧盟 GMP(包括无菌产品和片剂产品),世界卫生组织 GMP,澳大利亚 GMP 认证。
产品出口世界超过 160 个以上的国家。并参与了超过 5 个跨国药品技术转移项目和 70 个诊断试
剂的转移项目的质量保证工作,积累了丰富的药品/医疗器械生产的质量监管,委托生产监管,供
应商质量管理,研发和临床及注册的质量监管,上市后药物警戒的质量监管等经验。
Jessica Hu was graduated from China Pharmaceutical University in 1995. She has been worked
in Takeda, GSK, Beijing Novartis Pharama, Roche Dia and Baxter China, mainly in quality
control and quality assurance area. She is very familiar with worldwide GMP/GDP
requirements, including EU GMP/GDP(sterile products and tablet products), WHO
GMP/GDP, Australia GMP, ICH/PIC/S guideline, Korea GMP, Taiwan GMP/GDP and China GxP
related drug regulation requirements, as well as, ISO 13485, China MD GMP, US 21CFR part
820, MDR, IVDR related medical device regulation requirements. Lead the team successfully pass MHRA, WHO, TGA GMP inspection without major findings multiple times in past 21 years
working experiences in Pharma and 5 years working experiences in medical device area. Act
as quality workstream lead, successfully transferred 5 cross region drug products and more
than 70 IVD products. Have rich experiences in manufacturing supervision, CMO supervision,
supplier management, quality supervision in R&D, clinical trial, RA and PMS area etc.

茅亚超Robin
联合创始人 产品研发负责人Co-Founder and Head of Product Development at 华升智药科技开发(北京)有限公司Huasheng Pharmatech (Beijing) Co., Ltd.
茅亚超,华升智药科技开发(北京)有限公司的联合创始人、产品研发负责人,拥有丰富的工业互联网、ToB SAAS软件服务行业、百亿级日业务数据处理系统的架构、产品商业化经验。在加入华升智药之前,曾任慧策集团核心产品线负责人,其带领下的产品团队为ERP数字化领域带来了全渠道的业务解决方案。
作为CISAW(信息安全保障人员认证)的高级成员,他在工业互联网开发信息安全方面展现出了深入的专业知识。他同时也是国际制药工程协会(ISPE)的一员。茅亚超先生在北京大学光华管理学院 获得MBA学位,同时拥有计算机科学与技术专业的工学硕士学位。无论是在技术革新、产品开发还是团队管理方面,茅先生都展现出了他的专长和对卓越的不懈追求.
Robin Mao, co-founder and head of product development at Huasheng Pharmatech (Beijing) Co., Ltd., has extensive experience in industrial Internet, ToB SAAS software services, and the architecture and commercialization of systems handling daily business data on the scale of billions. Before joining Huasheng Pharmatech (Beijing) Co., Ltd, he was a key product line manager at HuiCe Group, leading product teams to introduce omnichannel business solutions in the ERP digitalization field. As a senior member of CISAW (Information Security Assurance Certification), he has demonstrated deep professional knowledge in information security within industrial Internet development. He is also a member of the International Society for Pharmaceutical Engineering (ISPE). Mr. Mao earned his MBA from Peking University's Guanghua School of Management and holds a Master of Engineering degree in Computer Science and Technology. Whether in technological innovation, product development, or team leadership and management, Mr. Mao has shown his expertise and relentless pursuit of excellence.

董立玮
亚太区首席合规顾问Principal Consultant APAC Compliance at 精鼎医药,Parexel International
董立玮在制药行业拥有超过15年从业经验,精通药品从研发到临床批次生产直至商业化全生命周期的法规符合,尤其关注中、美、欧制药法规框架,擅长质量体系建立与改进、官方检查和监管行动准备与应对、数据可靠性整改与提升、审计与有因调查等。2018年加入精鼎医药任战略合规咨询首席顾问,为全球制药和生物制药客户提供GMP相关咨询,包括协助客户通过各国官方检查、执行EU QP审计、执行或协助应对授权许可(License in/out)项目的合规尽职调查、提供培训等。在加入精鼎之前,立玮在诺华制药担任合规经理。立玮毕业于中国药科大学国家生命科学与技术人才培养基地,获生物工程学士学位。 Liwei Dong has over 15 years of experiences in the pharmaceutical industry. He is proficient in regulatory compliance of pharmaceutical products lifecycle from R&D to manufacturing of clinical batches till commercialized manufacturing, especially in the regulation framework of China, US, and EU. He excels at establishing and maintaining quality systems, preparing and responding Health Authority legal enforcements, remediating and improving data integrity, auditing and for-cause investigating etc. He joined Parexel in 2018 as a Principal Consultant in Strategic Compliance Consulting, and provides GMP consultation to pharmaceutical and biotech customers worldwide, including to support clients to pass various inspections, to conduct EU QP audits, to execute or defend due diligence audits in license in/out projects, and to provide trainings etc. Prior to join Parexel, Liwei worked as Compliance Manager in Novartis. Liwei has a bachelor’s degree on bioengineering at China Pharmaceutical University in the National Base of Life Science and Biotechnology Education.

谭炳合,Ph.D
副总裁 at 邦耀生物
谭炳合博士现为上海邦耀生物科技有限公司CMC副总裁,分管公司管线产品的CMC和生产制备工作。在细胞与基因治疗产品的开发和临床应用上积累多年实践经验,擅长使用新型基因编辑技术开发通用型细胞治疗技术&产品。以第一作者和共同作者身份在Nature、Advanced Science 、Cancer Research、Cancer Immunology Research等著名期刊上发表多篇研究论文。申请国际发明专利2项、中国发明专利4项,主持1项国家自然科学基金青年项目,作为技术骨干参与多项国家自然科学基金项目。目前正在负责多项CGT创新药的开发、制备、检验和注册申报工作,带领团队成功将三款细胞和基因治疗产品推进临床研究阶段。 Dr. Tan Binghe is currently the vice president of Shanghai BRL Medicine Co., Ltd., in charge of CMC and production of the company's pipeline products. He has accumulated many years of practical experience in the development and clinical application of cell and gene therapies, and is proficient in gene editing to develop advanced allogeneic-universal cell products. As the first author and co-author, he has published research papers in famous journals such as Nature, Advanced Science, Cancer Research and Cancer Immunology Research. He applied for 2 international invention patents, 4 invention patents in China, presided over a youth project of the National Natural Science Foundation, and participated in several projects of the National Natural Science Foundation as the key technician. At present, he is responsible for the development, preparation, inspection and registration of a series of CGT innovative therapies, and has successfully applied for IND approval for three CGT therapeutic products in China.

郭维强
上海血制 副总经理 Vice General Manager at 天坛生物 Tiantan Bio
国家一级建造师、医学生物工程师
First-class constructor/ Biomedical Engineer
主要项目经历:
成都蓉生永安血制项目(设计投浆1200吨,项目投资14.5亿) 项目经理
Chengdu Rongsen Yongan plasma facilities project (1200 ton/year
Plasma, investment 14.5 Billion) Projector
云南生物制品基地项目(设计投浆1200吨,项目投资16.5亿) 项目经理
Yunnan biological products facilities project (1200 ton/year
Plasma, investment 16.5 Billion) Projector
成都蓉生重组凝血因子项目(rhFVIII 26亿IU,项目投资5亿) 项目经理
Chengdu Rongsen recombinant FVIII facilities project (rhFVIII 26 Billion IU, investment 5 Billion) Projector
阿联酋Hayat疫苗工厂项目(项目投资3亿美金) 项目经理
UAE Hayat vaccinum facilities project (investment $16.5 Billion) Projector
主要擅长领域:
新建生物制品工厂项目全生命周期管理
Full Life Cycle Management of New Biological Products Factory Project
工厂预防性维护管理
Factory preventive maintenance management
设备设施验证
Equipment and facility validation

郭融 Willian
Vice President, R&D of Shanghai Innopac Medical Technology Co.,Ltd
William Guo, Vice President R&D for Shanghai Innopac Medical Technology Co.,Ltd.

王刚
执行董事、高级副总裁兼首席质量官 at 上海君实生物工程有限公司 Shanghai Junshi Biotechnology Co., Ltd
王刚博士现任上海君实生物执行董事、高级副总裁兼首席质量官,全面负责公司生产和研发质量及相关工作。加入上海君实生物前,曾任上海药明生物副总裁,负责质量和全球监管事务。王刚在美国 FDA 和中国 CFDA 工作 13 年,先后任 CFDA 药品审评中心 (CDE) 负责合规和检查的首席科学家,FDA CDER 合规办公室生产质量办公室资深政策顾问,FDA 驻华办公室助理主任,FDA CBER 合规和生物产品质量办公室资深审评员和主持检查员。王刚是经FDA 专业同行评定的生物产品 GMP 领域的专家,在单抗及细胞和基因治疗产品监管及质量管理方面有较高造诣。王刚本科毕业于南京大学生物化学专业,并获得美国 DartmouthMedical School 药理学与毒理学博士学位。博士毕业后在美国国家卫生研究院 (NIH) 国家癌症研究所 (NCI) 从事肿瘤免疫疗法领域的博士后研究,随后在美国德克萨斯州大学 MDAnderson Cancer Center 任助理教授和课题负责人。
Dr. Wang Gang is the Executive Director, Senior Vice President and Chief Quality Officer of
Shanghai Junshi Biosciences. At this role, he is responsible for the company's manufacturing
and R&D quality and related work. Prior to joining Junshi, Dr. Wang was the Vice President of
WuXi Biologics (Shanghai), responsible for quality and global regulatory affairs. Dr. Wang has
13 years of experience in the U.S. FDA and China's CFDA. He has served as Chief Scientist for
Compliance and Inspection in the Center of Drug Evaluation (CDE), CFDA; Senior Policy
Advisor in the Office of Manufacturing Quality in the Office of Compliance, CDER, FDA;
Assistant Country Director of the FDA China Office; senior reviewer and lead inspector in the
Office of Compliance and Biologics Quality, CBER, FDA. Dr. Wang is an FDA peer-reviewed
expert in the field of GMP for biologics, with expertise in regulation and quality management
of monoclonal antibody and cellular and gene therapy products. Dr. Wang graduated from
Nanjing University in biochemistry in China and received his PhD in pharmacology and
toxicology from Dartmouth Medical School in USA. He completed his postdoctoral research
in immunotherapy of cancer at the Surgery Branch of National Cancer Institute (NCI), National
Institutes of Health (NIH). He was an assistant professor and principal investigator at the MD
Anderson Cancer Center of the University of Texas prior to joining the FDA.

吴云飞
技术与法规经理 at Merck
目前负责支持默克BioReliance® 生物医药检测服务中国区的细胞基因治疗业务技术与法规事务。她曾专注于抗体类药物细胞培养板块工艺研发及生产。发表过多篇科学论文,专注于分子生物学。她拥有利物浦大学博士学位。
Yunfei Wu is responsible for supporting technology and regulatory affairs associated with BioReliance® Biosafety Testing Services in China, especially for cell and gene therapy. Yunfei worked in the process development of antibody-based therapy, especially for cell culture process development and manufacture. She published several scientific papers, focusing on molecular biology and graduated with a PhD from University of Liverpool.

曹晓平 Ph.D.
高级副总裁、技术运营部负责人 at 上海药明巨诺生物科技有限公司
曹晓平博士拥有超过21年在美国、中国和亚太地区的全球生物制药研发经验,成功支持了多款创新药物与疫苗在中国和美国的研发与上市,专长与经验包括新药开发、药学研究、生产、注册、及供应链管理等。
曹博士现任药明巨诺高级副总裁与技术运营部负责人,负责细胞治疗产品的工艺开发、技术转移、临床与商业生产、供应链及工程设施设备等。
加入药明巨诺之前,曹博士为辉瑞公司高级总监和全球药学中国负责人,组建并带领团队支持了辉瑞在中国的化学药、生物制剂及疫苗产品的药学注册申报,推动多个产品在华获批并支持产品生命周期管理。在此之前,曹博士曾在辉瑞美国和中国担任多个技术与管理职务,在制剂与工艺、药物材料科学、及分析技术方面积累了丰富经验与专长。
曹博士还曾担任默沙东中国研发药学负责人,组建并带领团队为临床试验申请、新药上市申请及上市后的药品提供药学支持,成功支持了多款关键药物与疫苗在中国的临床与上市批准。此外,曹博士还曾在位于新加坡的一家专注脑健康的创新药公司Cerecin担任副总裁、负责公司产品的药学研究、临床及商业生产与供应。
曹博士拥有威斯康星大学麦迪逊化学博士学位、康涅狄格大学MBA学位及清华大学化学学士学位。

陈四向
专家 at 广东东阳光药业股份有限公司
陈女士,制药行业超过三十年的工作经验,历任知名药企质量总监、总经理。曾负责从0到1到超百个产品获批及国内外商业化上市。带领团队通过二十余次的国内GMP检查,以及十余次的美、欧、WHO等国外GMP检查,有丰富的质量管理经验及国际GMP认证经验,也是新版GMP指南《口服固体制剂与非无菌吸入制剂》分册编委;曾与多家国内外知名的设计院合作,组织建设了四家工厂(含制剂及API)。在药厂建设,经营管理方面有丰富的知识和经验积累,是位认真负责,开拓创新的管理者。 Ms. Chen, with over thirty years of work experience in the pharmaceutical industry, she has served as the Quality Director and General Manager of well-known pharmaceutical companies. Promote the approval and commercialization of hundreds of new products. Lead team to pass the domestic GMP inspection for more than 20 times, as well as stringent regulatory authority (e.g. FDA, EMA, and WHO) GMP inspection for more than 10 times. Having rich experience in quality management and international GMP inspection, she is also a member of the editorial board of the new Chinese GMP Guideline "Oral Solid Preparations and Non-Sterile Inhalation Preparations". Collaborated with several well-known design institutes both domestically and internationally to organize the construction of four factories, including drug product and drug substance. With rich knowledge and experience in the construction and management of pharmaceutical factories, she is a conscientious and innovative manager.

陶静雯
量子创新场主任 at 明度智云
硕士毕业于英国牛津大学。现任职于明度智云量子创新场,主要负责人工智能在医药行业的应用探索和实施。研究方向包括药物研发、质量控制、生产过程以及合规性问题,涉及搭建AI药物属性预测平台、利用AI赋能的mRNA疫苗传递系统设计平台、AI药品生产异常检测平台以及AI图像检测平台,工作范围覆盖从算法设计到应用实践。
Graduated with a master's degree from the University of Oxford. Currently serves in the AI Innovation Department of Mingdu Smart Cloud, primarily responsible for the exploration and implementation of artificial intelligence-enabled applications in the pharmaceutical industry. Research areas include drug R&D, quality, production, and compliance scenarios, responsible for building an AI drug property prediction platform, an AI-enabled mRNA vaccine delivery system design platform, an AI drug production anomaly detection platform, and an AI image detection platform, covering everything from algorithm design to practical application.

韩亮
北京大学知识工程与监管科学实验室研究顾问Research Consultant, Knowledge Engineering and Regulatory Science Laboratories, BeijingUniversity at 识林知识平台负责人Head of Shlinx
韩亮博士,识林知识平台负责人,北京大学知识工程与监管科学实验室研究顾问。从事药品生产质量法规政策和产业质量体系
研究,牵头负责2010版GMP指南撰写和再版修订工作。长期参与IPEM项目的组织和教学。2014年毕业于北京大学。

马强
Sales Manager - Analytics at Knick (Shanghai) Electronic Measurement Trading Co., Ltd
马强毕业于上海大学测量技术与仪器专业,有10年的分析系统工程工作经验,在西门子、ABB、Servomex担任技术工程师和过程设计师,9年在科伲可、PR电子等公司担任分析仪和自动化产品的技术销售支持和销售。他在科伲可工作了4年,担任分析产品的应用工程师和销售经理,在产品应用和客户服务方面都有丰富的经验。
With bachelor degree of Measurement technique and instrumentation graduated from Shanghai University, Ma Qiang has: 10 years working experience for analytical system engineering as technical engineer and process designer in Siemens, ABB, Servomex. 9 years working experience as technical sales support and sales for analyzers and automation products in Knick, PR electronics etc. He has worked in Knick for 4 years as application engineer and sales manager of analytics products with lot of experience both for product applications and customer service.

黄玮
总裁 President of 上海复宏汉霖生物制药有限公司 Shanghai Henlius Biopharmaceutical Co.,Ltd.
黄玮女士做为公司总裁,全面负责生产与工程部全面负责商业化产品的生产供应及松江基地的建设,同时带领质量团队打造国际化质量标准体系和运行。
黄玮女士拥有华东理工大学生物化学工程学士学位及马里兰大学化学与生化工程硕士学位。在加入复宏汉霖之前,黄玮女士在制药和生物技术行业拥有30年的高级管理和领导经验,包括工艺开发、技术转让、制造、工艺和设施设计、资本项目执行和质量体系实施。
As president of the company, Wei leads the manufacturing, quality, MSAT, clinical supply, engineering, supply chain and other operations functions. Wei holds a Bachelor's degree in biochemical engineering from East China University of Science and Technology and a Master's degree in chemical and biochemical engineering from the University of Maryland. Before joining Henlius, Wei held several senior management and leadership positions in pharmaceutical and biotechnology industry. She has thirty years’experience in process development, technology transfer, manufacturing, GMP facility design, and quality system implementation.
Sponsors and Partners
This is a past event
国际制药工程学会(ISPE)上海代表处 might have other events you're interested in.
View more events


































